
Abstract
Aims: The aim of the study was to determine the effectiveness of a novel strategy to treat radial artery spasm (RAS).
Methods and results: We conducted a prospective, randomised, single-centre, open-label trial comparing a novel strategy of pressure-mediated dilatation versus intra-arterial administration of a combination of nitroglycerine plus verapamil for the treatment of RAS. The primary endpoint was radial artery intraluminal diameter acute gain assessed by quantitative radial angiography. After screening two hundred and twenty consecutive cases, twenty patients presented with RAS and were randomised 1:1 to either strategy. Overall the mean age was 60.8±11.5 years and 53% were females. Pre-treatment angiographic characteristics were similar between the groups. The primary endpoint of radial artery acute gain was significantly greater in the pressure-mediated dilatation group (0.85±0.46 mm vs. 0.03±0.24 mm, p<0.001). Blood pressure drop was significantly lower in the pressure-mediated dilatation group (ΔBP –3.8±24 vs. –31.6±19 mmHg, p<0.001). There was one case of radial artery occlusion in the pressure-mediated dilatation group at follow-up. Short-duration pain was observed during the application of pressure.
Conclusions: Pressure-mediated dilatation for the treatment of RAS was feasible, with superior angiographic results compared to a pharmacologic vasodilator strategy, with no impact on blood pressure. This novel approach proved to be safe and effective and should be tested in a large randomised trial.
Abbreviations
DVD: distal vessel diameter
MLD: minimal luminal diameter
PMD: pressure-mediated dilatation
PVD: proximal vessel diameter
RA: radial artery
RAS: radial artery spasm
RVD: reference vessel diameter
Introduction
Transradial access is rapidly becoming the access of choice for most coronary procedures worldwide1-3 due to reductions in vascular complications and major bleeding. In addition, emerging data suggest a decrease in mortality in patients presenting with acute coronary syndromes4-6. Nevertheless, vasoconstriction resulting in radial artery spasm (RAS) is a major limitation. RAS is reported in 2 to 34% of cases. It is associated with pain and, in severe cases, inability to perform the procedure further, this being the most frequent cause of access-site crossover7-9. A second arterial puncture not only increases the complexity of the intervention but is also a predictor of major vascular complications10.
To minimise the incidence of RAS, several studies have shown that downsizing of radial sheaths and catheters, use of hydrophilic coated sheaths, periprocedural sedation and pre-procedure administration of vasodilators are effective measures to prevent spasm8,9,11. However, the majority of these studies have focused on spasm prevention. In contrast, alternative treatments of spasm other than vasodilators have not been investigated. As increasingly more complex coronary interventions are performed through the radial artery (RA), treatment strategies for spasm resolution should be explored.
Based on the observation of radial artery dilation using retrograde manual injection angiography and founded on the theoretical basis of arterial wall circumferential stretch due to pressure overload, we investigated a novel strategy of pressure-mediated dilatation (PMD) compared to pharmacologic therapy to treat RAS12.
Methods
STUDY POPULATION
The study was a prospective, randomised, open-label trial performed at one centre comparing pressure-mediated dilatation vs. intra-arterial administration of a combination of nitroglycerine and verapamil. Between September and November 2015, two hundred and twenty patients underwent coronary angiography. After screening two hundred and twenty cases, we included 20 patients (9.0%) in whom the left radial artery could be successfully cannulated and where RAS subsequently developed, as confirmed by angiography (Figure 1). RAS was defined as a temporary, sudden narrowing of the radial artery lumen diameter, associated with acute pain, aggravated by the motion of the catheter. We excluded patients with known contraindications to verapamil or nitroglycerine (significant aortic stenosis, heart rate <50/min, high-grade atrioventricular block, myocardial infarction complicated by cardiogenic shock, left ventricular ejection fraction <35% or haemodynamic instability), patients with acute myocardial infarction and/or with renal insufficiency (creatinine [Cr] clearance <60 ml/min).
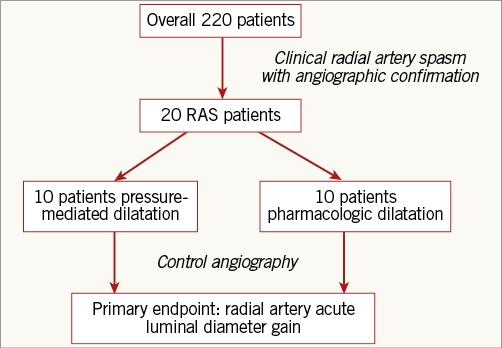
Figure 1. Study design. Patients with clinical and angiographic radial artery spasm were included. After randomisation to pressure-mediated dilatation or pharmacological treatment (verapamil+nitroglycerine) a second angiography was acquired to assess the primary endpoint.
PROCEDURE
After assessment of the left radial artery pulse, local anaesthesia was applied by subcutaneous injection of 0.5 ml lidocaine 2%. A prior hand circulation test (i.e. Allen’s test) was not mandatory13. Radial artery puncture was performed with a 21-gauge needle (4.0 cm Merit Advance®; Merit Medical, South Jordan, UT, USA). The arterial puncture was performed by a senior staff member or by a resident in training in interventional cardiology. A 0.021’’ non-hydrophilic guidewire was placed in the radial artery, and a 5 Fr arterial sheath (7.5 cm Avanti®; Cordis, Miami Lakes, FL, USA) was advanced. All patients received intravenous administration of unfractionated heparin, 5,000 U for diagnostic procedures and 70-100 U/kg for percutaneous coronary interventions.
Preventive administration of an intra-arterial vasodilator was not allowed. All procedures were performed using a 5 Fr sheath; if there were clinical signs of RAS during the procedure, a radial angiography was performed to confirm the spasm and then the patient was included in the study. For the PMD group, a controlled pressure algorithm using an angiographic injection system (Medrad Mark® V ProVis®; Medrad, Pittsburgh, PA, USA) with 10 ml of saline solution (0.9% sodium chloride; Baxter, Cali, Colombia) at a rate of 10 ml per second with a pressure of 400 psi through the radial sheath was used. In the pharmacologic group nitroglycerine 100 mcg plus verapamil 2.5 mg was infused through the radial sheath. The drugs were diluted in saline and administered slowly after aspirating some blood through the arterial sheath. Heart rate and blood pressure were recorded at baseline and 60 seconds after the application of either therapy. This was followed by a second radial angiography in the same projection. Pain in the forearm was assessed by a cathlab nurse trained to use an analogical scale graduated from 0 to 10 just after the procedures. A threshold of 7 defined a severe pain. No sedation or analgesia was used during any of the procedures. Haemostasis of the radial access was obtained with a simple gauze compression dressing for one to three hours. All patients were examined 24 hours after the procedure to assess for access-related complications. Doppler evaluation was performed at 24 hours and at 90 days. Antiplatelet treatment was left to the physician’s discretion before, during and after the procedure. All subjects provided written informed consent. The study protocol was approved by the Institutional Ethics Committee on Human Research and the study was conducted according to the principles of the Declaration of Helsinki.
Quantitative radial angiography analysis was performed for each patient by a blinded reviewer off-line with an automated edge-detection algorithm (CAAS 5.9.2 System; Pie Medical, Maastricht, the Netherlands) with the outer diameter of the sheath used as the calibration standard. We analysed RA segments 65 mm in length starting from the tip of the introducer. The following measurements were obtained: reference vessel diameter (RVD) using the interpolation method, 5 mm proximal vessel diameter (PVD) adjacent to the proximal region of interest, 5 mm distal vessel diameter (DVD) adjacent to the sheath, minimal lumen diameter (MLD), percent diameter stenosis (1–[MLD/RVD]×100) before and after the intervention, delta %DS (DS post–pre) (Figure 2). Acute intraluminal diameter gain was calculated (MLD post-intervention–MLD pre-intervention). RA to sheath ratio was calculated using the RA diameter in different segments divided by the diameter of the sheath before and after intervention. Treatment success was defined as residual diameter stenosis in the RA <30% with no angiographic evidence of dissection. Additionally, to evaluate the axial distribution of spasm, we divided each artery into three segments. Artery-to-sheath ratios were then reported for each subsection and compared before and after intervention. All images were analysed by two experienced operators blinded to treatment at an independent core lab (Cardialysis BV, Rotterdam, the Netherlands).
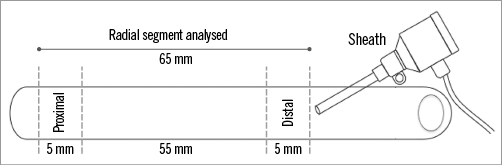
Figure 2. Anatomical landmarks for the quantitative radial artery angiography analysis.
STUDY ENDPOINTS
The primary endpoint of the study was radial artery diameter acute gain on quantitative radial angiography. Secondary endpoints included delta diameter stenosis (post–pre), treatment success, radial artery occlusion at follow-up, the occurrence of severe pain (≥7 on the analogic scale) in the forearm during the intervention (pressure or pharmacologic) and safety events (severe systemic hypotension, i.e., a decrease of >40 mmHg of systemic arterial pressure [SAP]; need for support of norepinephrine, atropine or fluids).
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±standard deviation or median (interquartile range [IQR]) and compared with a t-test or Mann-Whitney test as appropriate. Categorical variables are presented as counts and percentages and compared with the chi-square test. In this exploratory analysis, no sample size calculation was performed since this was the first application of this experimental treatment. Intraobserver and interobserver variability were assessed using intraclass correlation coefficients. All computations were performed with Stata 14.0 (StataCorp, College Station, TX, USA) and a two-sided p-value <0.05 was considered statistically significant.
Results
Twenty patients with RAS were randomised to be treated with PMD (n=10) or intra-arterial administration of nitroglycerine and verapamil (n=10). Overall, the mean age was 60.8±11.5 years, 35% were females, 30% were diabetics and the mean body-weight index was 26.0±SD kg/m2 (Table 1). Regarding procedure characteristics, access gained at the first puncture attempt, pre-intervention severe pain and the number of catheters used were similar between the groups. Pre-treatment radial angiography mean MLD and radial artery stenosis were 1.09±0.19 mm and 34% in the pressure-mediated group and 0.91±0.19 mm and 35% in the pharmacologic dilatation group (p=0.05 and p=0.94); artery-to-sheath ratio was greater in the PMD group, 1.04±0.21 vs. 0.86±0.14 (p=0.016) (Table 2, case examples Figure 3).
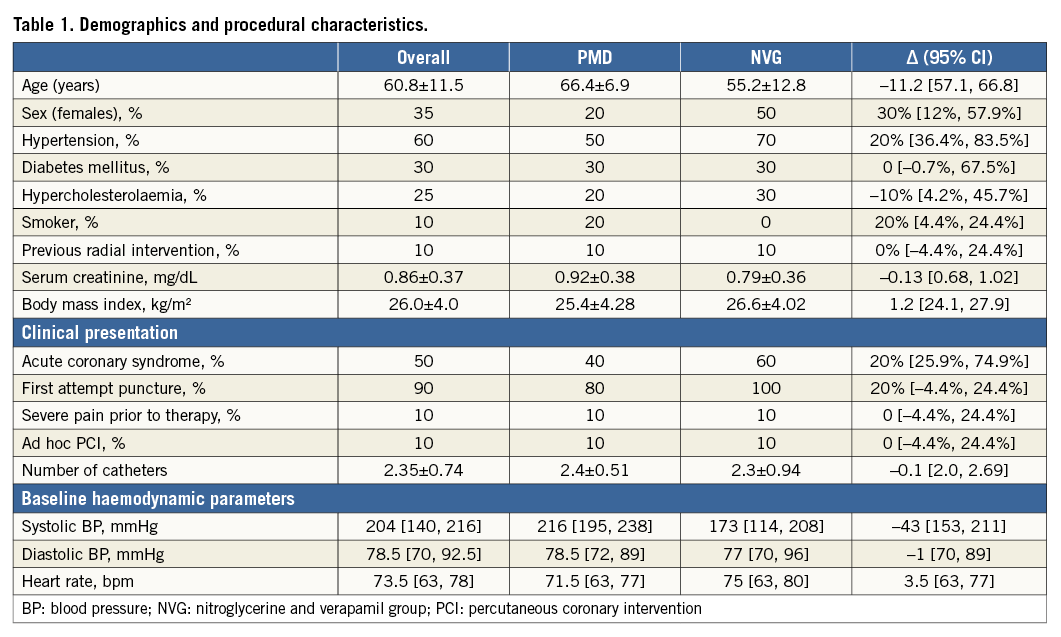
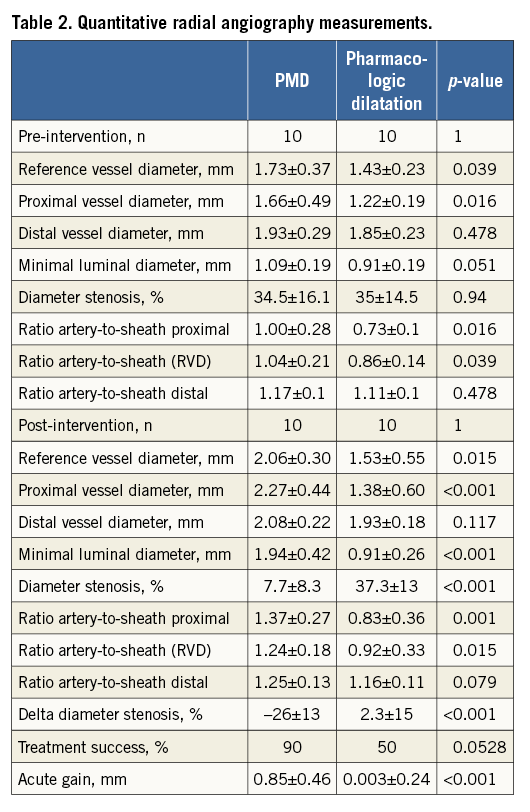
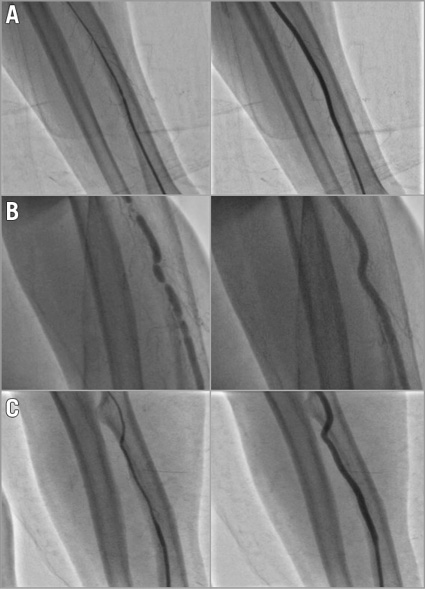
Figure 3. Three case examples of patients treated with pressure-mediated vasodilatation. On the left side, baseline angiography and, on the right side, control angiography with a significant improvement in angiographic spasm and significant increase in radial artery dimensions during different angiographic types of spasm. A) Diffuse radial artery spasm. B) “Rosemary” appearance spasm. C) Long diffuse radial spasm.
The primary endpoint of acute gain was significantly higher in the PMD group compared with the pharmacologic group (0.85±0.46 mm vs. 0.003±0.24 mm, p<0.001). Luminal diameter increased from 1.09±0.19 mm to 1.94±0.42 mm (p<0.001) in the PMD group vs. from 0.91±0.19 mm to 0.91±0.26 mm (p=0.97) in the pharmacologic group (Figure 4). Treatment success was 90% in the pressure-mediated dilatation group and 50% in the pharmacologic group (p=0.058); post-treatment percent diameter stenosis was significantly lower in the pressure-mediated dilatation group (7.7% vs. 37.3%, p=<0.001). There was no evidence of dissections in either group. RA-to-sheath ratio increased in the PMD group, with no change after nitroglycerine and verapamil administration; however, RA-to-sheath ratio only increased using medium and proximal vessel diameter (proximal segment p=0.001, medium segment p=0.006 and distal segment p=0.203) (Figure 5). The intraclass correlation coefficient using MLD measurements for interobserver agreement was 0.98 (95% CI: 0.96-0.99; p<0.001) and 0.94 (95% CI: 0.86-0.97) for intraobserver agreement.
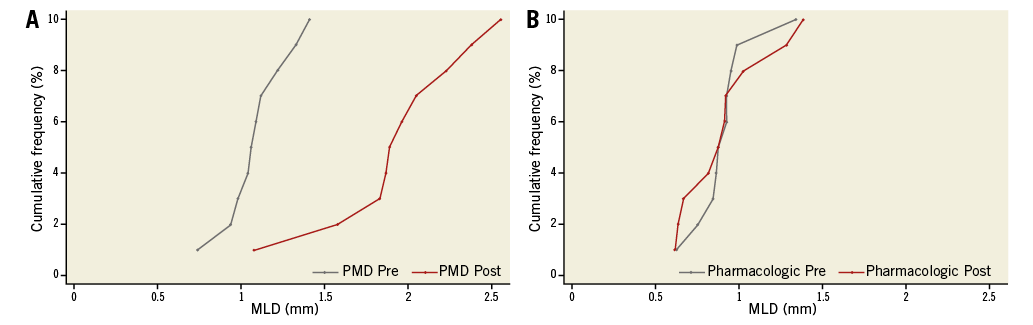
Figure 4. The cumulative curves illustrate the immediate effect in minimal lumen diameter (MLD) of pressure-mediated vasodilatation compared with intra-arterial pharmacologic treatment as assessed by quantitative radial angiography. PMD: pressure-mediated vasodilatation; Post: after intervention; Pre: before intervention
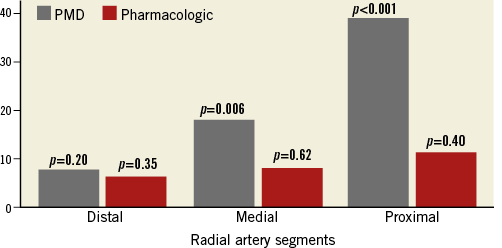
Figure 5. Representation of changes in artery-to-sheath ratio (as percentage) distributed among portions of the radial artery. In the pressure-mediated group (grey), a significant increase in artery-to-sheath ratio was noticed in the middle and distal segments; however, no significant increase was found in the intra-arterial pharmacologic treatment group (red) at any segment.
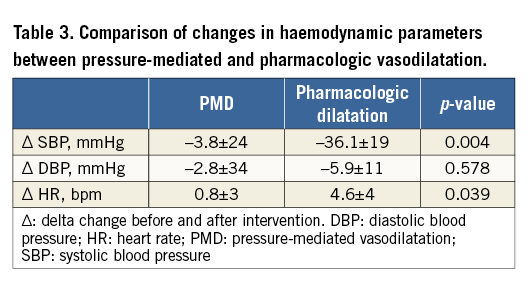
Radial artery patency was assessed with Doppler ultrasound in all patients 24 hours and 90 days after the procedure. There was a single case of early RA occlusion in the PMD group. No other access-related complications were observed. Severe pain was more common during the application of pressure (90% vs. 10%, p<0.001), whereas haemodynamic compromise with severe hypotension occurred more frequently in the pharmacologic dilatation group (10% vs. 50%, p=0.004) (Table 3). One patient required fluids administration due to severe symptomatic hypotension after the administration of verapamil and nitroglycerine.
Discussion
The present study is the first to evaluate a strategy of pressure-mediated vasodilatation for the treatment of RAS. This novel technique proved to be safe and superior to the administration of intra-arterial pharmacologic vasodilators, with no concerns regarding a blood pressure drop.
Continuous progress in coronary interventions is shifting the choice of arterial access to the radial artery. Reliable evidence on improvement in vascular complications with a repercussion in terms of mortality has been found in contemporary trials14. However, in a small subset of patients, this benefit is lost due to RAS. In the RIVAL study, 5% of patients underwent access-site crossover due to the inability to treat spasm4. Therefore, there is a need for new treatments or strategies to overcome this limitation.
The main histological feature of the radial artery is a thick tunica media with a high vasospastic activity in response to constrictor stimuli15. Transradial access and cardiac catheterisation precipitate media contraction and spasm. Vasoconstriction in the RA has been shown to respond to vasodilators. Using isolated radial artery segments, He et al demonstrated that nitroglycerine and verapamil are active agents in preventing arterial spasm16. Additionally, Boyer et al investigated the effects of intra-arterial vasodilators on radial artery size, assessed by radial angiography before catheterisation. An increase in radial artery diameter after the administration of intra-arterial nitroglycerine and verapamil was found17. Our study included patients with clinically and angiographically confirmed spasm and found no significant increase in RVD or MLD after the administration of nitroglycerine and verapamil. The use of vasodilators was associated with a significant systemic blood pressure drop. This was not seen in the PMD group, making this novel technique useful even in haemodynamically unstable patients. Interestingly, the overall median systolic blood pressure was 204 (140, 216) mmHg, which could be a consequence of the pain occurring during RAS.
Concerning arterial patency, recent studies have suggested pharmacological or compression measures to prevent RA occlusion18-20. Although asymptomatic, radial occlusion precludes the utilisation of the radial artery as an access site for future procedures, coronary bypass grafting or haemodialysis. Therefore, strategies to minimise RA occlusion should be implemented and patency evaluation must be performed after transradial procedures as a quality maintenance strategy, as suggested by the Society for Cardiovascular Angiography and Intervention’s Transradial Working Group21. In our study, Doppler evaluation was performed 24 hours after the intervention with no concern regarding RA occlusion. There was one arterial occlusion in the PMD group at early follow-up; however, since this technique could eventually produce a stretch-induced injury, long-term patency analysis should be performed. Severe pain was noticed in all patients during the application of pressure. Nevertheless, this was of short duration, lasting only for the few seconds of the injection (one to two seconds).
A possible mechanism of vasodilatation using pressure is the strain on the radial artery created by pressure overload in the vessel wall which causes mechanical distension. The unique characteristics of the RA facilitate the accommodation of an acute increase in pressure, restoring RA dimensions without angiographic evidence of vessel injury. Recently, a balloon-assisted tracking technique was described to treat severe RAS using an inflated balloon partially protruding through the distal end of the catheter. Thus, a mechanical principle to overcome vasoconstriction was effectively proven22. The fact that vessel dimensions are restored after retrograde pressure provides an insight into the mechanism of vasoconstriction underlying RAS where a combination of media contraction and vessel collapse appears to coexist. Also, the vasodilation observed with PMD could be related to the activation of the stretch-activated calcium channels in the endothelium which increases the production of nitric oxide (NO), a known potent vasodilator. The per-segment analysis using a sheath-to-artery ratio evidenced a more pronounced spasm in the proximal segment of the analysed artery (60 mm from the tip of the introducer). This finding is consistent with what was found when analysing radial artery spasm with optical coherence tomography23. The distance between the tip of the introducer and the smaller diameter in a spastic artery may explain the lack of vasodilatation observed with the administration of nitroglycerine and verapamil through the sheath.
We have described a simple, inexpensive and fairly well-tolerated novel technique to reverse RAS. From the angiographic point of view, this method was successful in 90% of the cases and was particularly useful in patients with contraindications for vasodilators or with borderline blood pressure. This study provides a new option for the treatment of severe radial artery spasm that may have an impact on the success of transradial interventions.
Strengths and limitations
The main strength of this trial is that a new therapy was tested, with no prior reports in the literature. It was conducted in a randomised fashion with an independent core lab angiographic analysis. The present study has some limitations. First, since this exploratory analysis aimed to assess the efficacy and safety of this novel approach, no sample size calculation was performed and a small number of patients were included in the trial. This warrants future investigation in a larger sample randomised controlled trial. Second, the assessment of diameter stenosis in spasm evaluation is limited due to difficulties in the measurement of reference vessel diameter. For that reason, DS was assessed as a secondary endpoint in the trial. Lastly, intravascular imaging to assess vessel injury was not used (e.g., dissections, intimal tears), although optical coherence tomography (OCT) studies have shown radial artery damage even with normal appearance angiography22. In addition, a clear concern with this method is the risk of local complication secondary to high-pressure injection at the radial site; however, we did not find any acute (i.e., dissection) or late (i.e., radial artery occlusion) vascular complication in this population. Nonetheless, larger trials should be performed to assure the safety of this method.
Conclusions
Pressure-mediated dilatation for the treatment of RAS was feasible, with superior angiographic results compared to a pharmacologic vasodilator approach. There was no impact on blood pressure, heart rate or evidence of vessel injury. Short-duration pain was more frequent during the application of pressure.
Guest Editor
This paper was guest edited by Mamas Mamas, DPhil, FRCP, BM BCh; Keele Cardiovascular Research Group, Keele University, Keele, United Kingdom.
| Impact on daily practice Radial artery spasm is the most frequent complication of transradial catheterisations despite the efforts to prevent its occurrence. Pressure-mediated dilatation (PMD), performed by a pressurised injection through the arterial sheath, showed restoration of the radial artery dimension, resolving the radial spasm without any safety concern. Furthermore, PMD was found to be superior, compared to a pharmacological approach, regarding angiographic spasm resolution. In daily practice, PMD might be used as an alternative treatment in cases of radial artery spasm. This technique might be particularly useful in patients with haemodynamic instability. |
Conflict of interest statement
P.W. Serruys and Y. Onuma are members of the Advisory Board for Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

