Abstract
Aims: To assess: the reasons behind an operator choosing to perform radial artery catheterisation (RAC) as against femoral arterial catheterisation, and to explore why RAC may fail in the real world.
Methods and results: A pre-determined analysis of PREVAIL study database was performed. Relevant data were collected in a prospective, observational survey of 1,052 consecutive patients undergoing invasive cardiovascular procedures at nine Italian hospitals over a one month observation period. By multivariate analysis, the independent predictors of RAC choice were having the procedure performed: (1) at a high procedural volume centre; and (2) by an operator who performs a high volume of radial procedures; clinical variables played no statistically significant role. RAC failure was predicted independently by (1) a lower operator propensity to use RAC; and (2) the presence of obstructive peripheral artery disease. A 10-fold lower rate of RAC failure was observed among operators who perform RAC for >85% of their personal caseload than among those who use RAC < 25% of the time (3.8% vs. 33.0%, respectively); by receiver operator characteristic (ROC) analysis, no threshold value for operator RAC volume predicted RAC failure.
Conclusions: A routine RAC in all-comers is superior to a selective strategy in terms of feasibility and success rate.
Introduction
Radial artery catheterisation (RAC) is an increasingly employed technique for percutaneous coronary interventions worldwide1. The steady increase in the use of RAC in recent years is rooted in increased patient comfort, the potential of reduced hospital stays, and the reduced rates of complications relative to femoral artery catheterisation (FAC)2-4. As a possible consequence of this, we and others have reported strikingly better clinical outcomes associated with the use of RAC in multicentre observational studies5-9.
Notwithstanding, RAC is a demanding technique that requires advanced technical skills developed via a steep learning curve10. Accordingly, success rates vary according to the expertise of the operator2,3,5. Moreover, different operators employ different criteria to select patients who will undergo RAC versus FAC, mostly based upon clinical variables and their own personal previous experience. All these factors have resulted in the inconsistent use of RAC across different centres, but also between different individual operators, which has blunted the potential benefits of this technique from a community point of view. Increased awareness of the factors influencing the choice of which arterial access to use by any given operator and the issues associated with RAC success may allow us to anticipate, prevent or overcome procedural difficulties, thereby, further improving outcomes. Unfortunately, to date, no study has sought to identify these factors formally in a large cohort of unselected patients undergoing RAC by operators with different levels of expertise and across centres with varying volumes of activity.
Therefore, we conducted a sub-analysis of data gleaned from the PREVAIL study, a large observational multicentre trial of consecutive patients undergoing any percutaneous cardiovascular procedure requiring arterial access, in order to assess (a) the reasons behind the choice of one arterial access site versus another, primarily RAC versus FAC, and whether these motivations not to perform radial approach were realistic and (b) the reasons for failed RAC in real-world cardiology practice where different physicians operate on unselected populations of patients.
Methods
A more detailed description of the methods used in the PREVAIL study has been published elsewhere5. In brief, this study was the first, large, prospective, observational trial to obtain a specific “snapshot” view of access site-related outcomes (local vascular complications, bleedings, acute myocardial infarction/re-infarction and death) for percutaneous interventions in contemporary cardiology practice. To obtain this snapshot perspective, all data were collected within a one month time window, across nine centres that were selected to be representative of Italian healthcare: three of the centres were high-volume centres that perform >2,000 cardiology procedures per year; three were moderate-volume centres (between 1,000 and 2,000 procedures/year); and three were low-volume centres (<1,000 procedures/year). Among those centres, 5/9 (55%) usually performed RAC in their everyday practice. Overall, 42 operators participated in the study, among whom, 16 (38%) reported a high volume of radial procedures (e.g., performing > 65% of their caseload by RAC in the year preceding the study).
We enrolled 1,052 consecutive patients who underwent an invasive procedure requiring arterial access in the catheterisation laboratory. Patients were excluded if they were enrolled in other research protocols requiring specific therapy or a specific arterial access site. The choices of arterial access site and the technique employed were made by individual practitioners, according to their usual practice.
The pre-specified aims of this PREVAIL study sub-analysis were 1) to assess the reasons behind the choice of one arterial access site versus another, and their influence on radial approach success on an intention to access basis; and 2) to identify the influence of operator and centre characteristics on the previous factors. Indeed case report forms were structured to appraise specific variables which identify: (a) the reasons for any arterial access choice as described and specified by the relevant operator, (b) centre and operator characteristics (as given by the head of the relevant catheterisation laboratory before the study) and the procedural characteristics (as given by the relevant operator) which might be linked to arterial access success. The reasons of the choice were categorised with a questionnaire encompassing pre-specified classes of answers. Blank spaces could be filled by the relevant operator only if the reasons of choice did not fit into the categorisation. Only 126/1052 (12%) of these blank spaces were filled and the ensuing results were categorised post hoc.
The failure of any arterial approach was defined as the need for a second, alternate, approach needing to access a different limb artery after the first attempt. The reasons for any arterial approach failure were also specified by each operator and recorded in all case report forms.
Statistical analysis
Continuous variables for each of the two subject groups, patients who underwent FAC as a first arterial access procedure and patients who underwent RAC as a first arterial access procedure, were reported as means with standard deviations; categorical variables were reported both as absolute numbers and percentages. Continuous variables were compared using independent-samples Student’s t or Mann-Whitney U tests, where appropriate. Categorical variables were compared by means of Pearson’s chi-square analysis or Fisher’s exact test, where appropriate. Multivariable binary logistic regression analysis was performed to appraise the independent predictive role of selected study variables on substudy outcomes. The selection of the variables for the final multivariable model was performed using a backward stepwise algorithm. These results are reported as odds ratios (OR) with associated 95% confidence intervals (CI).
Receiver operator characteristic (ROC) analysis was performed as an exploratory evaluation to identify the best cut-off points for radial access activity volume for each operator to predict RAC success.
A p value of <0.05 was considered statistically significant, with all reported tests 2-tailed. All statistical analyses were performed using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Centres, operators and procedural characteristics
Detailed population characteristics of PREVAIL study have been previously published5. Further data relevant to this analysis are presented in Table 1.
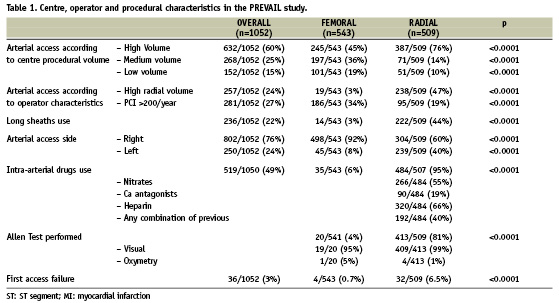
High-volume centres enrolled the majority of patients in both groups; however, in the RAC group, a statistically higher proportion of procedures was performed at high-volume institutions, relative to the FAC group. Similarly, high-volume radial operators more often performed the procedure by RAC, while operators who performed > 200 percutaneous coronary interventions per year more often utilised the femoral approach. Patients undergoing RAC received sheaths that were, on average, longer but of lesser diameter than those undergoing FAC. Overall, an Allen test was performed on 81% of RAC patients and the right arm was the preferred limb in this group. Vasodilator drugs were administered intra-arterially in 95% of RAC patients, but only 40% received these drugs in a combination with a spasmolytic cocktail.
Patients undergoing RAC more often needed an arterial access site change than their FAC counterparts.
Arterial access choice
Overall 509/1052 patients (48%) were selected to undergo RAC. The reasons operators gave for choosing either RAC or FAC are shown in Table 2. Overall, the operator’s personal preference was the reason given for 90% of procedures, while only 10% of patients had the site of arterial access selected on the basis of specific clinical considerations. This pattern was similar for RAC and FAC patients; however, compared to RAC, operators more often chose FAC because they perceived a clinical contraindication for another approach. The other reasons that led to operators choosing FAC included subclavian stenosis, obesity, the use of 7-9 Fr sheaths, cardiogenic shock, active dialysis with an arterio-venous fistula, the need for right heart catheterisation, and patient non-compliance with the radial approach. Meanwhile, clinical reasons for choosing RAC included anticoagulation, an abdominal aortic aneurysm, and the patient request for radial approach.
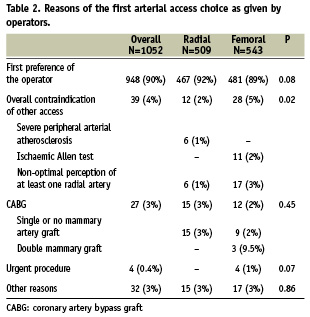
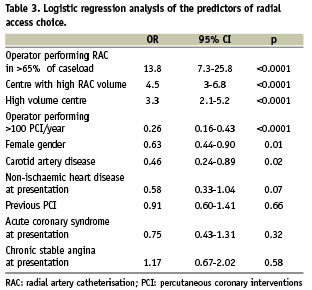
Predictors of arterial access choice identified by multivariate analysis are shown in Table 3. Independent predictors of RAC for first access were (1) having the procedure done by an operator who performs > 65% of his/her personal caseload by RAC; and (2) undergoing the procedure at a high RAC volume or a high overall procedural volume centre. Conversely, women and patients with carotid artery disease were less likely to be selected for RAC, as were patients undergoing the procedure by operators who perform > 100 coronary interventions per year.
Success with the radial approach
Overall, a second, different access site had to be used in 6.5% of patients (Table 1). Left and right radial approaches had similar failure rates (17/205 vs. 16/304; 8% vs. 5%, respectively. P=0.17).
The reasons for RAC failure were unfavourable vascular anatomy in 44% (14/32) of patients, arterial spasm in 28% (9/32), unsuccessful puncture in 19% (6/32), dissection of the radial artery in 3% (1/32), and other unspecified technical problems in 6% (2/32). No statistically significant differences were observed in the reasons for failure between left and right radial approach. Similar failure rates were observed in centres with high overall versus low overall procedural volumes. However, at high RAC-volume centres, a statistically lower rate of failure was observed than at low RAC-volume centres (Figure 1). In accordance with this, the cohort of patients who experienced successful first arterial access procedures had that procedure performed by operators with a statistically higher percentage of cases performed via the radial artery than patients requiring a second access site (80±19% vs. 68±28%, respectively; p=0.02). Moreover, the failure rate for first radial access decreased by almost 10 fold, from 33% to 3.8%, when operators with < 25% of their caseload performed by RAC were compared against those with > 85% of their personal caseload performed by RAC (Figure 2). However, ROC analysis failed to detect any significant threshold value for RAC volume, by operator, to obtain successful first arterial access (Figure 3).
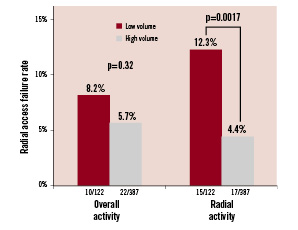
Figure 1. Rates of radial access failure relative to medical centre procedural volume. Patients undergoing RAC at hospitals with high overall procedural volumes exhibited a similar rate of RAC failure as those undergoing the procedure at low-volume hospitals. However, assessing RAC volumes alone, patients undergoing the procedure at high-volume hospitals experienced a statistically lower rate of RAC failure that their low-volume hospital counterparts.
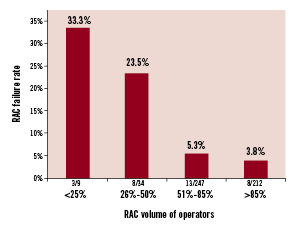
Figure 2. Rates of radial access failure relative to operator RAC procedural volume. A 10-fold decrease in the rate of radial access failure was observed among operators who use RAC for more than 85% of their caseload versus those who use it less than 25% of the time.
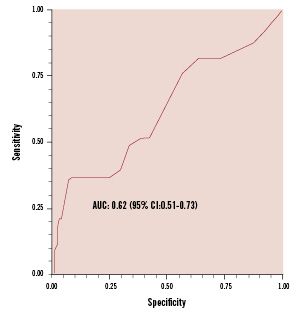
Figure 3. ROC analysis of the association between operator RAC procedural volume and success rate. No significant threshold for operator RAC procedural volume was identified that predicts RAC failure. AUC: area under the curve by extended trapezoidal rule; CI: confidence interval
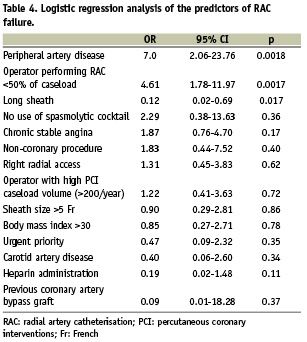
Predictors of radial access failure on multivariate analysis are shown in Table 4. Independent predictors of RAC failure were the presence of peripheral artery disease and, consistent with the previous analysis, having the procedure done by an operator who uses RAC for < 50% of his personal caseload. Conversely, using a long arterial sheath was associated with an increased rate of RAC success.
After RAC failure the most commonly choosen access was femoral (29/36, 81%), while omeral access was used in 2/36 patients (6%) and only 5/36 patients (23%) underwent the procedure through the contralateral radial artery.
Discussion
For the first time in a large real world setting, the PREVAIL study yields specifically sought insights into how centre, operator and patient-related characteristics influence the choice of arterial access site and the rate of success of the arterial access procedure.
The first original finding of our analysis is that the choice of first arterial access site was due to operator preference in 90% of cases, while perceived clinical or technical factors affected the choice in only a small minority. This translated, on multivariate analysis, into a major predictive role of operator experience and propensity for the choice of RAC for first access. Therefore the choice of a given arterial approach mostly depends upon the discretion of the operator, rather than on insurmountable patient characteristics. More specifically, our study showed that regardless of the reasons given by operators, multivariate analysis revealed that only female gender and carotid artery disease were negative predictors of initial RAC. Therefore the question arises whether the reasons commonly believed convincing not to perform radial approach may be realistic, considering that the statistically significant factors at multivariable analysis have also been overcome. Indeed, women appear to experience a higher RAC failure rate, but also significantly greater outcome benefits with radial access than men; hence, routine RAC may be preferable for females3. Still, the radial approach sometimes is considered dangerous in the presence of carotid artery disease, because of the potential exposure of diseased arteries to catheters and wires; on the other hand, a number of randomised and observational reports have failed to show any association between RAC and stroke2,5,7. Urgent procedures (including treatment of acute myocardial infarction), higher calibre sheaths and catheters (e.g., 7 and 8 Fr), double mammary grafts and left/right heart catheterisation can be managed by RAC11-15,18. Additionally, some authors have suggested that the Allen test is unhelpful at predicting the safety of RAC, so this test may not be adequate to select suitable patients16,17. In fact, we identified as many as 96 RAC (19% of the total) performed in the absence of any prior Allen test, and these procedures generally yielded good outcomes.
Secondarily, consistently with previous literature2,3,5, our data show that RAC success in the real world is largely dependent upon operator experience. However a quantification of this relationship was still missing. We could show an almost 10-fold decrease in failure of first access when the procedure is performed by someone who uses RAC in >85% of their caseload relative to those who use it infrequently. In fact, though prevalent among subjects in the PREVAIL study, the role of anatomic or functional variables (e.g., arterial tortuosity and radial spasm) seem to be prevented effectively by the use of long arterial sheaths. Indeed, we showed for the first time that the use of long sheaths is the only technical factor significantly and independently associated with increased RAC feasibility, rendering local variables like spasm and unfavourable anatomy irrelevant to predictions of RAC feasibility in real cardiology practice. Whether this was due to a high prevalence of operators unfamiliar with radial approach still remains to be ascertained in specific studies. Our data also failed to pinpoint any significant cut-off value in operator procedural volume which predicts greater success with RAC. This suggests that there is no minimal level of RAC experience that ensures procedural success; in other words, the higher an operator’s RAC procedural volume, the better his or her rate of success tends to be.
In conclusion and in contrast to what is generally believed true by interventionalists, for the first time our study provides the evidence that the routine use of RAC in general percutaneous cardiovascular interventions is feasible, safe and more successful than its selective use. Pooling the results of this substudy with those of the main PREVAIL analysis imply that the generalised, routine use of radial artery catheterisation has the potential to enhance real-world clinical outcomes previously demonstrated with this access.
Limitations of the study
A discussion of PREVAIL study limitations has been already published5. Additionally this subanalysis endeavoured to discriminate the very complex matter of individual operator biases and motivations, therefore, the categorisation of the reasons of access choice could have been limited and therefore leading to an additional error in the analysis. However the fact that only 12% of motivations needed to be completed by additional description testify to the quality of the pre control of confounders.
Moreover, not only this was a secondary analysis where the sizing always is inappropriate, but also the study was a limited snap shot that picked a limited number of centres to represent a broad base of practices. However all the efforts have been done to select appropriate centres that reflected Italian real-world practice according to the official records of the Italian interventional cardiology society5. Also, operators were balanced in the fact that approximately half of the patients undergoing radial approach were performed by high radial volume operators. Nevertheless, low-volume operators may well have been on a learning curve and this in turn could have affected the ROC analysis results in the fact that no cut-off in personal volume was found. Even so, the purpose of this analysis was to analyse contemporary “real-world” situations, which includes a patchy distribution of high radial volume operators across centres and operators on a learning curve. Also, the fact that operators with greater skill are more likely to use the radial access as a default approach may have biased the feasibility analysis based on arterial choice, but it is also true that climbing the learning curve itself by selecting more often any radial approach is the main cause of greater skill, therefore the study conclusions are likely to be still considered valid.
Finally, the low prevalence of patients undergoing radial procedure by operators performing radial approach in < 50% of the times may have affected the incidence of radial failure. However the very high incidence of radial failure in these patients makes the hypothesis unlikely.
Acknowledgements
The PREVAIL study group was composed as follows:
Chairmen: Christian Pristipino, MD; Giuseppe Richichi, MD.
Investigators:
S. Filippo Neri Hospital, Rome (Antonino Granatelli, MD; Giulio Speciale, MD; Vincenzo Pasceri, MD; Francesco Pelliccia, MD; Adriana Roncella, MD; Maurizio Turturo, MD);
Catholic University of the Sacred Heart, Rome (Carlo Trani, MD; Francesco Burzotta, MD; Antonio Maria Leone, MD; Mario Attilio Mazzari, MD; Rocco Mongiardo, MD; Giampaolo Niccoli, MD; Antonio Giuseppe Rebuzzi, MD; Italo Porto, MD; Antonella Tommasino, MD; Filippo Crea, MD);
San Camillo Hospital, Rome, (Roberto Violini, MD; Marco S. Nazzaro, MD; Francesco De Felice, MD; Rosario Fiorilli, MD; Antonio Parma, MD; Maurizio Menichelli, MD; Edoardo Pucci, MD);
Sant’Andrea Hospital, Rome, (Andrea Berni, MD; Biagio Andrea Pace, MD; Domenico Maria Zardi, MD; Roberta Coluccia, MD);
Campus Biomedico University, Rome, (Germano Di Sciascio, MD; Giuseppe Patti, MD; Andrea D’Ambrosio, MD; Marco Miglionico, MD; Rosetta Melfi, MD; Annunziata Nusca, MD);
Policlinico Casilino Hospital, Rome, (Ernesto Lioy, MD; Roberto Patrizi, MD; Francesco Summaria, MD; Alessandro Sciahbasi, MD);
Madre G. Vannini Hospital, Rome, (Bruno Pironi, MD; Salvatore Donato Musarò, MD; Igino Proietti, MD; Gaetano Canale, MD);
San Carlo Hospital, Rome, (Pietro Mazzarotto, MD; Francesco Gemelli, MD);
Sant’Eugenio Hospital, Rome, (Achille Gaspardone, MD; Gaetano Gioffrè, MD; Carlo Citone, MD).

