
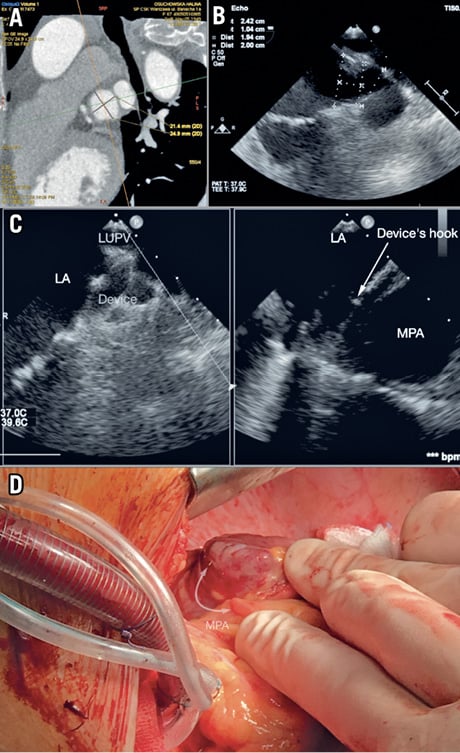
Figure 1. Preprocedural, intra-interventional and surgical views of LAA/occluder. A) & B) LAA sizing on CT TEE. C) LAA/occluder/MPA on TEE. D) Intraoperative view of the occluder’s anchors and MPA perforation site.
Major bleeding is a rare complication of percutaneous left atrial appendage closure (LAAC)1. One of the causes of bleeding is main pulmonary artery (MPA) perforation which may occur not only in a few hours after LAAC but can also happen up to 17 days after2,3.
A 67-year-old patient with paroxysmal atrial fibrillation (PAF) qualified for LAAC due to gastrointestinal (GI) and intracranial bleeding when treated by novel oral anticoagulant (NOAC)/oral anticoagulant (OAC) therapy.
The patient’s history showed an ischaemic stroke, diabetes type 2, rheumatoid arthritis (RA) treated by prednisone and radioiodine hyperthyroidism therapy. The CHA2DS2-VASc score was calculated at 9.8%. Based on pre-op computed tomography (CT) and transoesophageal echocardiography (TEE), the left atrial appendage (LAA) was identified as windsock type and the landing zone (LZ) dimension was assessed as 20 mm (Figure 1A, Figure 1B). The MPA close to the LZ was dilatated up to 31 mm. A 2 mm free space between the MPA and the LAA was found. An AMPLATZER™ Amulet™ 25 mm device (St. Jude Medical [now Abbott], St. Paul, MN, USA) was implanted during sinus rhythm (SR), without additional manipulations and recaptures. The procedure was successful and uneventful; no pericardial effusion (PE) was observed (Figure 1C).
Seventeen hours after LAAC, the patient reported acute chest pain and dyspnoea. Echocardiography revealed massive, fast progressive pericardial effusion.
Intraoperatively, a small injury of the MPA (3 mm, latero-posterior wall) was found. The MPA cut was caused by the occluder’s anchors, which perforated the LAA LZ (Figure 1D). Suture repair of the MPA with a PROLENE® 5-0/Teflon patch was performed. The LAA was dissected. The patient was discharged seven days after surgery.
A 62-year-old man with a history of colitis ulcerosa (CU) and PAF qualified for LAAC due to recurrent GI bleeding on NOAC/OAC therapy. The CHA2DS2-VASc score was calculated at 2.2%. Due to having had CU, the patient had been treated with sulfasalazine for 10 years. Based on pre-op TEE, the LAA was identified as a windsock type and the LZ was assessed as 23 mm.
The MPA close to the LZ was 24 mm by TEE. Between the LAA/MPA no free space was found. An AMPLATZER Amulet 28 mm device was implanted during SR, without additional manipulation or recapture. The procedure was successful and uneventful. No PE was noted. Three hours after LAAC, clinical signs of tamponade were observed. During pericardiocentesis, 500 ml of arterial blood was evacuated. Following interventional drainage, the patient was haemodynamically unstable; progressive PE was found in echo. During surgery, several injuries of the LAA caused by device anchors were identified and a 3 mm tear of the latero-posterior wall of the MPA was found. The tear was sutured with PROLENE 4-0/patches. The LAA was dissected. Seven days after surgery the patient was discharged.
In summary, both of our patients were treated with immunosuppressive and anti-inflammatory drugs and had SR during implantation. Additionally, reduced space between the MPA and LAA walls was noted. We assume that all of the above features may be considered as potential risk factors for device anchor-related MPA perforation after LAAC.
Conflict of interest statement
P. Scisło reports receiving a travel grant from Abbott. The other authors have no conflicts of interest to declare.

