A 69-year-old male with paroxysmal atrial fibrillation discontinued anticoagulation due to gastrointestinal bleed. He suffered an embolic stroke in 2006 while off anticoagulation. Warfarin was resumed after this event but was stopped again due to recurrent gastrointestinal bleed requiring blood transfusion. Due to high risk of stroke and anticoagulation intolerance, he underwent successful percutaneous LAA closure with the LARIAT suture (SentreHEART, Inc., Redwood City, CA, USA) in June 2012 (Online Figure 1, Moving image 1). A six-week follow-up TEE revealed late recanalisation of the LAA with a patent channel measuring 4 mm in diameter at the suture line (Online Figure 2, Moving image 2). Follow-up TEE at three and six months documented a persistent 4 mm channel at the ostium of the LAA. Due to high risk of stroke and significant persistent flow into the LAA, we proceeded with repeat LAA closure. Because of concerns of possible entanglement of a new LARIAT delivery system with the previously placed LAA suture, we decided to use a different device. We chose an off-label use of an AMPLATZER™ septal occluder (ASO) device (St. Jude Medical, St. Paul, MN, USA). We surmised, due to the narrow neck at the suture site, that it would provide a good seal with enough anchoring at the waist to prevent embolisation. The patient underwent successful repeat LAA closure in December 2012 with a 10 mm ASO device (Figure 1, Moving image 3). Initially we deployed a 6 mm ASO device but this was not stable in the LAA. Due to concern of embolisation, we used a 10 mm ASO (Figure 1, Moving image 3). To our knowledge, this is the first reported case of a successful repeat percutaneous LAA closure with an ASO device for late partial recanalisation of the LAA after a prior successful suture with the LARIAT device. This approach is feasible and could be considered when incomplete closure of the LAA is found after surgically or percutaneously placed LAA epicardial suture.
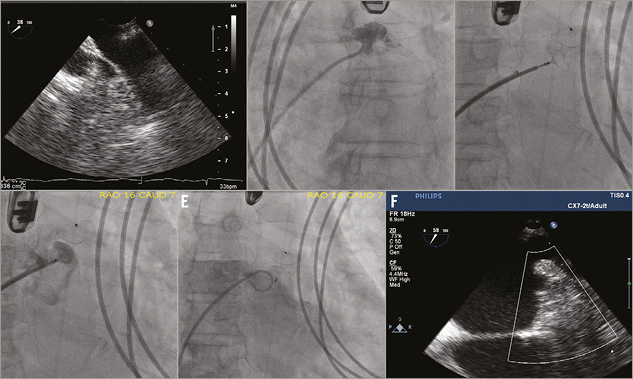
Figure 1. A) Patent LAA visualised by TEE with 4 mm diameter at ostium. B) LAA angiogram revealed patent LAA. C) ASO device being deployed in the LAA. D) LA angiogram demonstrated proper position of the ASO device without peri-device flow prior to device release. E) Repeat LA angiogram after ASO device was released in the LAA revealed adequate closure. F) Post-procedure TEE confirmed complete LAA closure without residual leak. ASO: AMPLATZER septal occluder; LA: left atrium; LAA: left atrial appendage; TEE: transoesophageal echocardiogram
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix. Discussion
Both surgical and percutaneous left atrial appendage (LAA) closure have received a Class IIb indication in the 2012 focused update of the European Society of Cardiology Guidelines for the management of AF. However, complete LAA closure is not always achieved. Incomplete closure rates have been reported to be as high as 30-70% for the surgical exclusion of the LAA using sutures or staples1-4. Persistent flow is also frequent after percutaneous LAA closure.
In the PROTECT AF study, up to 32% of the patients had peri-device flow at one-year follow-up5. The clinical significance of incomplete LAA closure is unknown. LAA thrombus was found to be present in 41% of the patients with unsuccessful LAA exclusion. However, no difference in stroke rate was found among patients with successful closure versus incomplete closure3. For patients at very high risk of recurrent stroke, a repeat attempt at LAA closure might be considered necessary. The indication for repeat LAA closure at this time may be weak due to lack of evidence. However, it may be beneficial for some patients and its role should be evaluated. The choice of closure device will depend on the patient’s anatomy and the mechanism of the residual leak. In this case, recanalisation of a prior successful LAA closure was seen with subsequent persistent flow at the suture line. The mechanism of recanalisation is not completely understood. It is possible that local oedema during the procedure might have prevented flow across incomplete closure, and flow became present once the oedema resolved over time. Another possible explanation may be that the LARIAT became loose over time. We demonstrated that LAA closure with an ASO device is feasible after incomplete closure with the LARIAT suture. This approach could be considered when residual leaks through the ostium of the LAA are seen after surgically or percutaneously placed LAA epicardial suture.
Online Figures
Moving image 1. A) TEE at baseline prior to LAA closure demonstrates a patent LAA. B) Left atrial angiogram at baseline corroborates patency of LAA. C) TEE immediately post-LARIAT suturing reveals a successful LAA closure without evidence of colour Doppler flow across the LAA. D) LAA angiogram post-LARIAT suturing confirms adequate LAA closure without residual flow into LAA.
Moving image 2. Six-week follow-up TEE reveals late recanalisation of the LAA with evidence of flow by colour Doppler across the 4 mm channel at the suture line.
Moving image 3. Successful repeat LAA closure. A) Patent LAA visualised by TEE with 4 mm diameter at ostium. B) LAA angiogram revealed patent LAA. C) ASO device being deployed in the LAA. D) LA angiogram demonstrated proper position of the ASO device without peri-device flow prior to device release. E) Repeat LA angiogram after ASO device was released in the LAA revealed adequate closure. F) Post-procedure TEE confirmed complete LAA closure without residual leak.
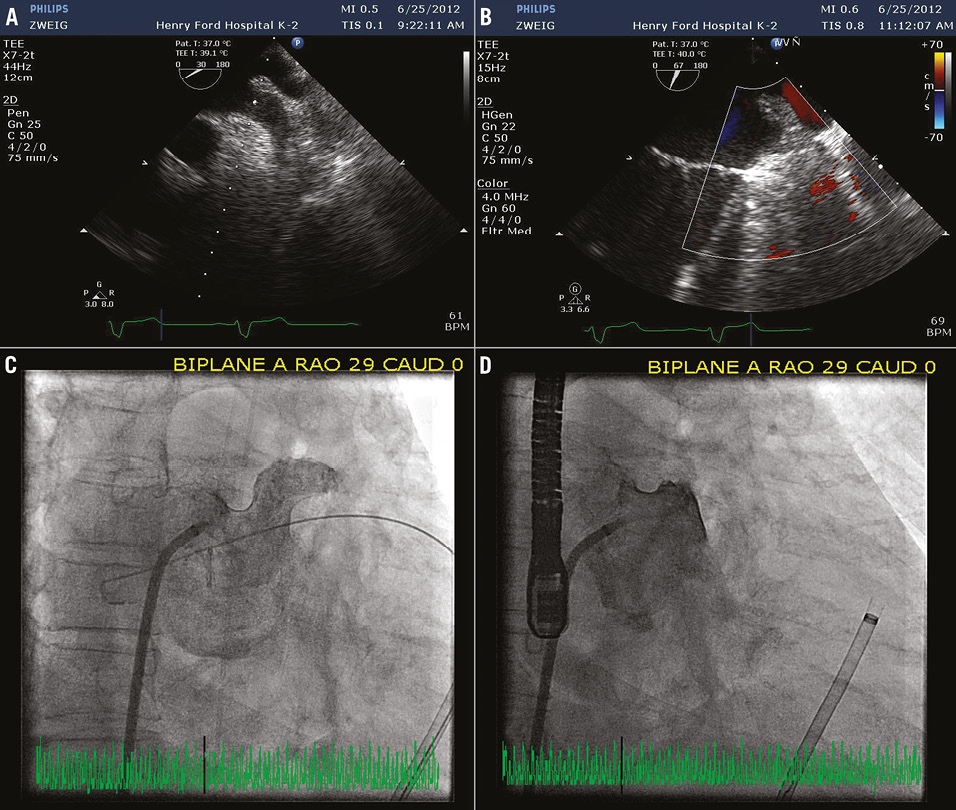
Online Figure 1. A) TEE at baseline prior to LAA closure demonstrates a patent LAA. B) Left atrial angiogram at baseline corroborates patency of LAA. C) TEE immediately post-LARIAT suturing reveals a successful LAA closure without evidence of colour Doppler flow across the LAA. D) LAA angiogram post-LARIAT suturing confirms adequate LAA closure without residual flow into LAA. LAA: left atrial appendage; TEE: transoesophageal echocardiogram
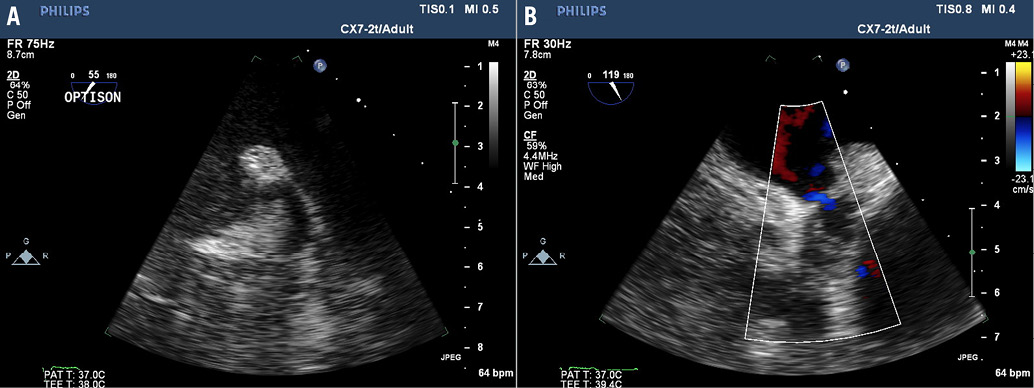
Online Figure 2. Six-week follow-up TEE reveals late recanalisation of the LAA with evidence of flow by colour Doppler across the 4 mm channel at the suture line.

