
Abstract
Aims: Aortic arch atresia (AAA) is one of the rarest obstructive defects. The presence of this anomaly in adult age is uncommon. The typical anatomic feature consists of a complete occlusion of the membranous obstruction resulting in an acquired atresia without flow continuity between the proximal and distal segments. This feature is important in determining the feasibility of percutaneous intervention. The aim of the present study was to share long-term follow-up data of adult patients with AAA requiring percutaneous interventions for the management of this rare anomaly involving five different centres.
Methods and results: Retrospective data of 19 patients (12 males, 63.2%, mean age 32.2±18.9 years) diagnosed with AAA treated in five different centres between 1999 and 2017 were collected. All patients underwent percutaneous recanalisation by (1) radiofrequency (RF) system (five patients, 26.3%), (2) extra-stiff guidewire (12 patients, 63.2%), and (3) transseptal needle (two patients, 10.5%). All procedures were subsequently followed by covered stent implantation. Two patients developed complications during the procedure and one of them died. Over a median follow-up of 4.94 years, four (21%) patients were able to be weaned from medications for hypertension. All the patients underwent reassessment for recurrence or restenosis during the follow-up. Seven (36.8%) patients underwent successful stent dilatation with a balloon. After the intervention, one patient experienced a late complication; however, one patient died due to an unknown cause believed to be unrelated to the previous recanalisation procedure.
Conclusions: Percutaneous treatment of AAA is feasible with good long-term survival. This study reports the largest case series so far available in the literature.
Introduction
Aortic arch atresia (AAA) is a rare congenital obstructive defect and is considered to be an extreme form of the coarctation of the aorta (CoA). The presence of AAA in adulthood is uncommon. The typical anatomic feature consists of luminal continuity between the aortic arch and descending thoracic aorta through an atretic segment, which appears like a fibrous cord. It differs from an interrupted aortic arch in which luminal continuity does not exist1,2.
Adult patients affected by AAA are usually asymptomatic due to the presence of extensive collateral circulation. However, the diagnosis is usually established during clinical workup of systemic arterial hypertension or claudication3. Clinical sequelae are similar to those encountered in aortic coarctation, e.g., untreatable systemic arterial hypertension, stroke, heart failure, aortic dissection, coronary artery disease (CAD) and premature death4.
The surgical management of AAA remains challenging in adult age due to extensive collaterals in the chest wall leading to difficult dissection and bleeding. It usually requires isthmus patch plasty or extra-anatomical conduits to manage the obstructive segment. Moreover, surgery is also associated with paradoxical hypertension, stroke, paraplegia, laryngeal nerve palsy and perioperative mortality4,5. Therefore, percutaneous interventions have recently been offered as an alternative, less invasive and effective option. In the available literature, only a few anecdotal case reports are available describing outcomes after percutaneous interventions of AAA1,6,7,8,9,10,11. Thus, the aim of the present study was to share long-term follow-up data of adult patients with AAA requiring percutaneous interventions for the management of this rare anomaly involving five different centres around the globe12.
Methods and materials
A multicentre, retrospective cohort study was performed on all adult patients who were diagnosed with AAA and underwent a percutaneous recanalisation procedure of the involved segment with covered stents between the years 1999 and 2017. The study was conducted in compliance with the Good Clinical Practices protocol and the principles of the Declaration of Helsinki. Institutional review board approval was obtained according to the respective institutional protocols. Demographic and clinical data were acquired from clinical databases.
The procedure was performed under general anaesthesia with endotracheal intubation and full heparinisation. Invasive systolic blood pressures were measured simultaneously at a proximal and distal site to the atresia at the beginning of the procedure. The recanalisation of the aortic atresia was performed by a radiofrequency (RF) system (Baylis MedComp, Montreal, Canada) using the stiff end of a guidewire (Cross-IT 300 [Guidant, Indianapolis, IN, USA], ACS BMW 0.0014” [Abbott Vascular, Santa Clara, CA, USA], GLIDEWIRE® Hydrophilic Coated Guidewire [Terumo Corp., Tokyo, Japan], Platinum Plus™ 0.018” [Boston Scientific, Marlborough, MN, USA]) or by transseptal needle. The RF system, consisting of a Nykanen 0.024” RF guidewire (Baylis Medical, Montreal, Canada) and a coaxial catheter, was advanced inside either a multipurpose or a right Judkins coronary artery catheter which was placed from below or from above the atresia. The catheter course was decided depending upon the patient’s anatomy with the aim of directing the wire towards the interrupted segment. Energy of up to 20 “watts” (W) was delivered for three to five seconds, repeatedly, allowing a smooth advancing of the RF wire and a perforation of the tissue between the proximal and distal aortic lumens. When a guidewire was used (0.014-inch coronary guidewire), the stiff end was pushed forward, perforating the tissue between the proximal and distal lumens. In all the cases, predilation of the intended site was performed using coronary artery or peripheral angioplasty balloons. The stents and the balloons were chosen according to the diameter of the transverse or aortic distal arch. Final angiographic imaging was performed after the procedure to show forward flow across the covered stent and patency of the left subclavian artery. All the patients were hospitalised for 48-72 hours after the procedure and received prophylactic antibiotics for 24 hours. They were directed to take aspirin (3 mg/kg to 5 mg/kg once a day) for six months after the implantation.
During the follow-up, all patients underwent (1) a physical examination, that included blood pressure measurements in the upper and lower limbs, an electrocardiogram and echocardiography at 1, 3, and 12 months post procedure and yearly thereafter, (2) ergometric tests at 3 and 12 months postoperatively and then yearly thereafter, and (3) computed tomography (CT) scans at 12 months post procedure. Statistical analyses were performed using SPSS statistical software, Version 20 (IBM Corp., Armonk, NY, USA). Data are presented as number (percentage) or mean±standard deviation.
Results
DEMOGRAPHICS
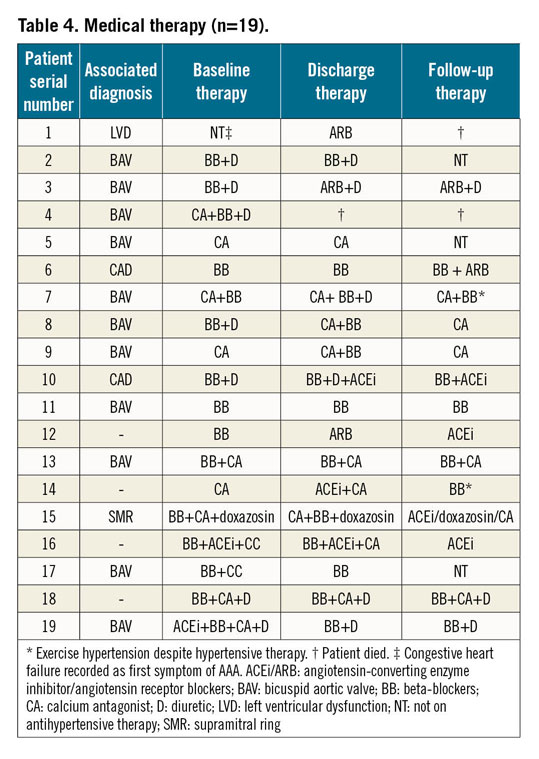
Nineteen adult patients with AAA (male/female: 12/7, 63.2% male) from five different centres (four from Europe and one from the United Arab Emirates) were identified. The median age and weight at the time of stent implantation were 36.32±18.9 years (range 30-60 years) and 67.68±13.39 kg (range 56-80 kg), respectively. The average body mass index was 24.2±3.73 kg/m2. On admission, 18 (94.7%) patients were on antihypertensive medications. Five patients (26.3%) were receiving three medications, six (31.5%) were on two and seven (36.8%) patients were taking single medication for control of blood pressure.
The median right arm systemic blood pressure among the patients was 150/90 mmHg (range 120/80-220/120 mmHg). Eleven patients (57.8%) had bicuspid aortic valve in association with AAA. Two patients (10.5%) had coronary artery interventions and one of them had severe left ventricular dysfunction. One patient (5.2%) had previous surgical correction of the supravalvular mitral ring. None of the patients had an associated feature suggestive of any genetic or chromosomal anomalies including Marfan’s or Turner’s syndrome. The initial diagnosis of AAA was suspected clinically due to the presence of systemic hypertension refractory to therapy in all the cases; the majority of them were in New York Heart Association (NYHA) Class I-II (Table 1). The final diagnosis was confirmed by transthoracic echocardiography (TTE) or with other imaging tools such as a cardiac CT scan or magnetic resonance imaging (MRI).
RECANALISATION PROCEDURE
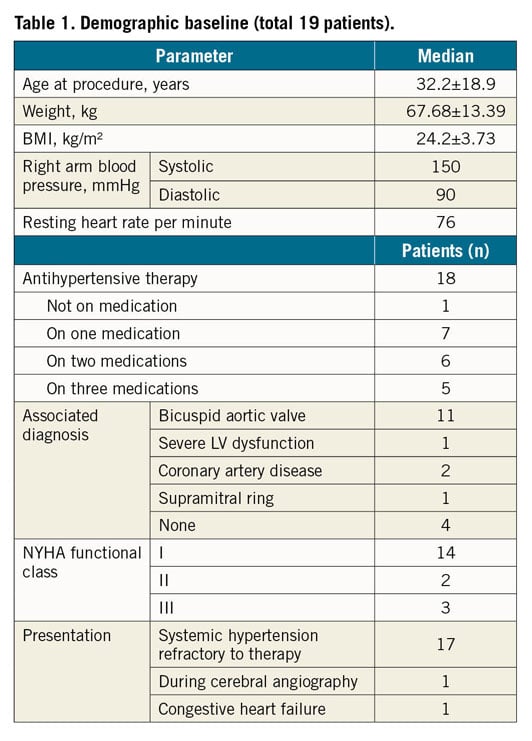
All subjects received 5,000–7,000 IU of heparin and antibiotics intravenously. For catheterisation, femoral arterial access was used in all the patients. However, for catheterisation of the proximal segment of the aortic atretic lumen, an additional radial artery access was obtained in 13 of these patients and a brachial access was obtained in six patients. Simultaneous angiography in the ascending and descending aorta confirmed the presence of complete occlusion at the aortic isthmus in all of the cases (Figure 1). The median length of the atretic segment was 10 mm (range 4-26 mm). In addition, median pre- and post-atretic segment systolic pressures were 138/125 mmHg and 88/85 mmHg, respectively. Twelve patients (63.2%) were treated with the stiff end of a guidewire pushed forward to perforate the tissue in the atretic segment of the isthmus. Unfortunately, this approach caused irreversible haemorrhage and shock leading to death in one patient. Five patients (26.3%) were treated with antegrade perforation using the RF system (average/median energy used: 18/18 W). In this group, one patient suffered from transient ischaemia of the left arm, which was resolved within the hospitalisation period. In the remaining two patients (10.5%), a Brockenbrough needle was used for antegrade perforation without any complications (Table 2). A concluding angiogram confirmed the absence of contrast leak and adequate intraluminal positioning in all cases (Figure 2). In order to achieve antegrade flow, the lesion was sequentially dilated using coronary artery or peripheral angioplasty balloons.
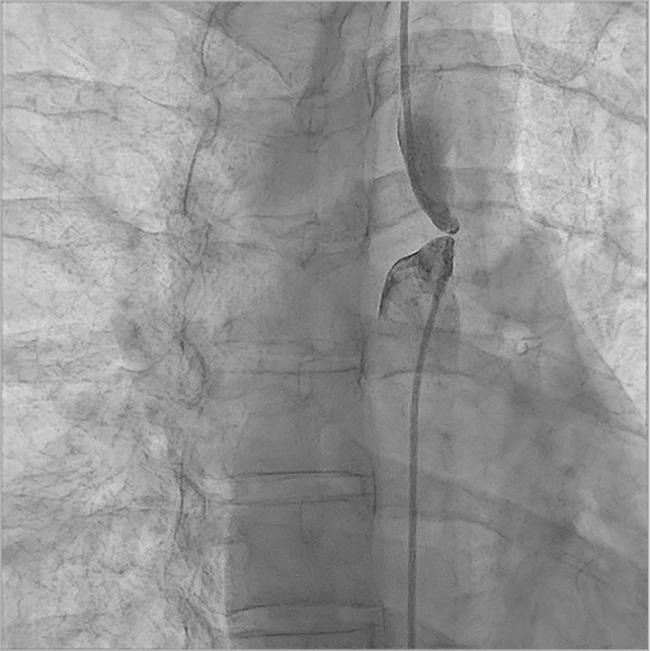
Figure 1. Angiogram in anteroposterior projection. Contemporary angiogram in both pre and post AAA confirms the presence of complete occlusion at the aortic isthmus.
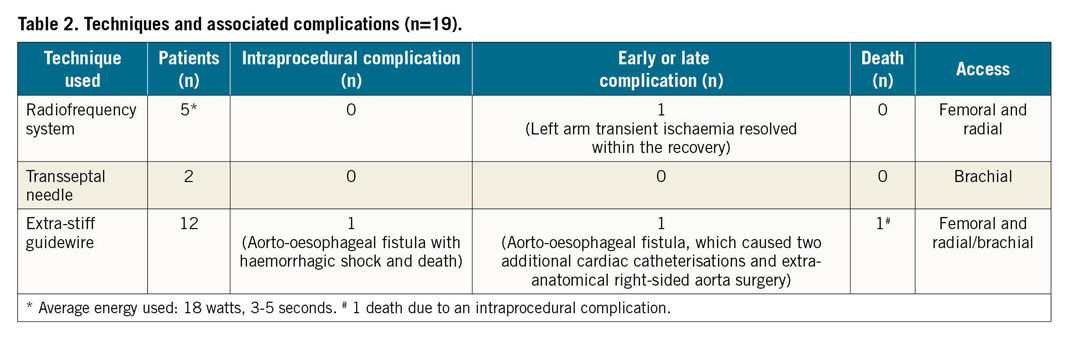
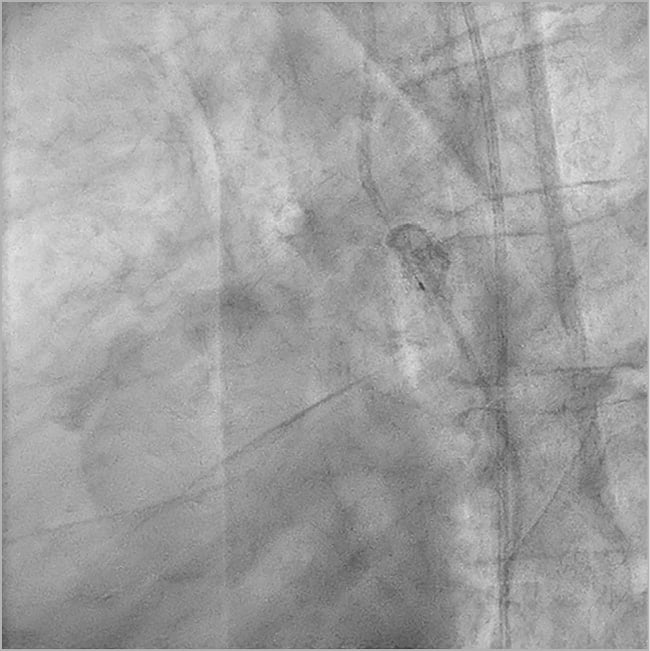
Figure 2. Angiogram in left lateral projection. The RF guidewire passed from the upper to the lower segment. The contrast injection confirmed the correct position.
An arterio-arterial circuit was created in all the patients between the femoral and the brachial/radial artery and a 0.035-inch Amplatz Super Stiff™ guidewire (Boston Scientific) was then exchanged over the circuit in order to place a 12 Fr to 14 Fr Mullins long sheath (William Cook Europe ApS, Bjaeverskov, Denmark).
Stent length (range 29-45 mm and median 45 mm) and balloon diameter (range 10-20 mm, median 15 mm) were selected according to the diameter of the descending aorta at the level of the left subclavian artery. The different types of stent used during the procedures are reported in Table 3. During this phase of the procedure, the balloon was manually inflated and the stent was successfully implanted in the correct position. Concluding angiography was then performed after placement of the stents that had shown good results and ruled out a dissection or rupture of the aorta in all the cases (Figure 3A). Any residual gradient was assessed after the procedure and a final angiography scan confirmed anterograde flow across the covered stent and the patent left subclavian artery (Figure 3B). Eighteen (94.7%) patients were discharged home within five days on antihypertensive therapy despite good results of the procedure. None required intensive care admission.
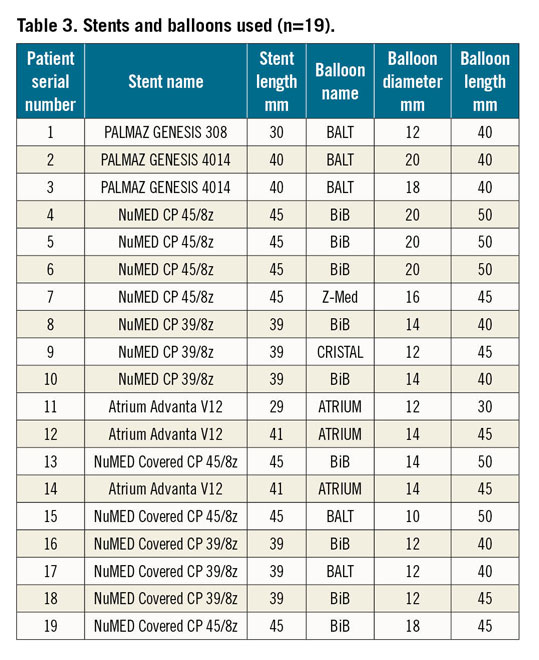
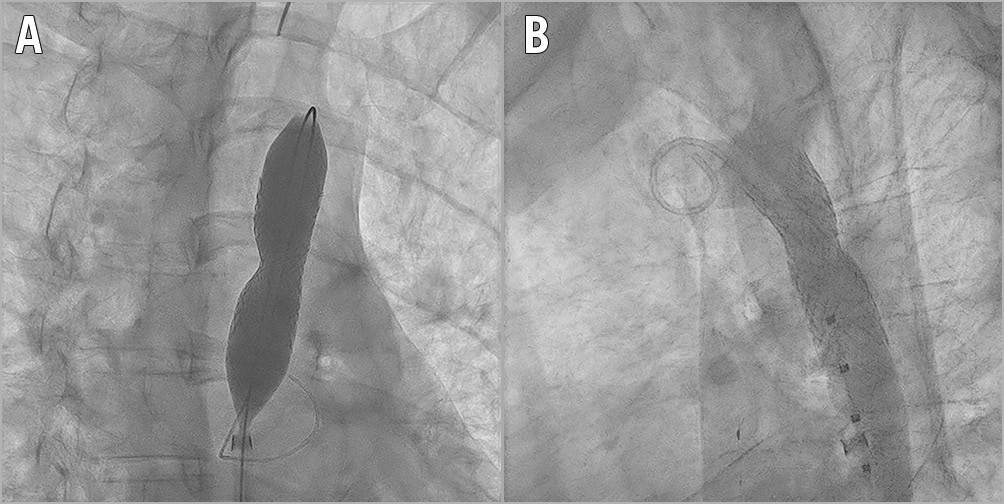
Figure 3. Angiograms in two different views. A) Right anterior oblique view: an Atrium Advanta V12 stent was positioned. B) Left lateral view: final result. No evidence of dissection. Stent fully expanded.
FOLLOW-UP ASSESSMENT
To date, we have collected data from the last follow-up visit for each patient after the procedure (with a median interval of five years [range 11 months-9 years]) (Figure 4).
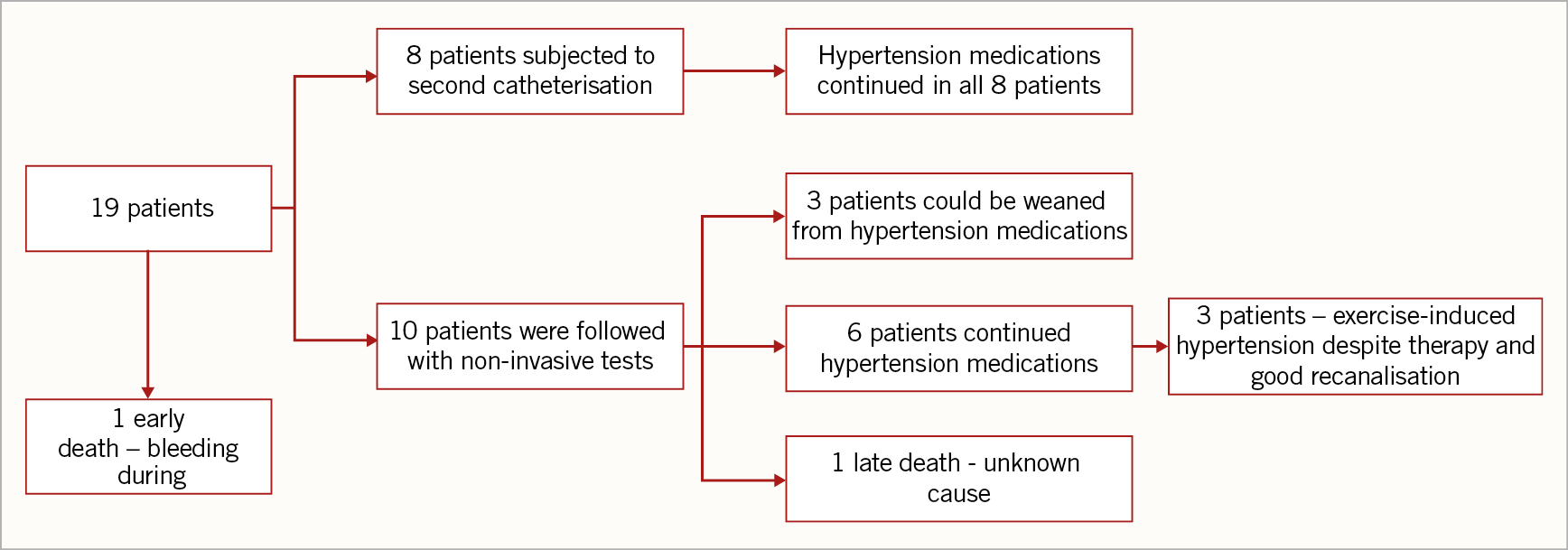
Figure 4. Follow-up.
One patient died during the follow-up (11 months after the procedure) due to an unknown cause, possibly related to left ventricular failure. Unfortunately, no post-mortem examination was carried out and therefore we are not able to provide more details. In addition, one patient had a late complication: after the discharge he underwent a second cardiac catheterisation to implant a second stent (Atrium Advanta V12, 14×29 mm; Getinge, Merrimack, NH, USA) because of a malalignment between the original stent and the axis of the aorta. One year later, he developed acute chest pain, secondary to a contained rupture of the aorta. An urgent thoracic endovascular aortic repair (TEVAR) was performed with placement of a stent (Endurant™ II stent graft, 16×20 mm; Medtronic, Minneapolis, MN, USA) and an aortic endoprosthesis extension with a GORE® EXCLUDER® 23 mm (W.L. Gore, Flagstaff, AZ, USA). After two years, an aorto-oesophageal fistula was revealed and an emergent TEVAR was performed by covering the aortic leak (proximal to the previous prosthesis) with a GORE® TAG®, 21 mm×10 cm endoprosthesis (W.L. Gore) in the aortic arch and extending into the previous prosthesis. Due to the persistent patency of the oesophageal fistula, a surgical procedure was undertaken (replacement of the aortic segment with a homograft and repair of the oesophageal fistula). Later, due to another massive bleeding, another emergent endograft placement was required. Finally, the homograft was surgically removed and an extra-anatomical right-sided aorta was surgically created using a prosthetic conduit13.
In eight patients, a second cardiac catheterisation was performed after a median period of 16 months. The indications of reintervention were presence of stenosis in two patients (as detected by echocardiographic or CT scan evidence of stenosis) or persistent hypertensive therapy in six patients during routine centre-specific follow-ups.
Seven of these patients were addressed with balloon re-dilation of the involved aortic segment because of demonstrable significant intra-stent restenosis (Figure 5). The average/median balloon diameter used to re-dilate the previous stent was 15.2/15 mm (range 12-18 mm). Among the patients who required redo interventions, none of them was able to be weaned from antihypertensive therapy.

Figure 5. Second re-dilatation.
In patients with a successful first intervention, exercise hypertension was recorded during follow-up in three patients despite good recanalisation and antihypertensive therapy (Table 4). However, all such patients were in NYHA functional Class I.
Discussion
Interruption of the aortic arch can be considered to be at the extreme end of the spectrum of obstructive thoracic aortic anomalies that begin with coarctation. This anatomic condition is a challenging clinical problem. The surgical management of such cases can be hazardous due to the presence of extensive collateral circulation in the chest wall that can cause bleeding and pose a high risk of paraplegia after the procedure14. Alternatively, a transcatheter option includes a balloon dilatation with or without stenting of the involved segment of the aorta. The mechanism of relief of obstruction involves mechanical stretching and a controlled intimal tear8,9,10,11,12,13,14.
The present study reports outcomes of a relatively large cohort of patients undergoing percutaneous treatment for AAA. It also supports the concept that it is technically feasible and safe to recanalise a local atresia of the aortic isthmus using catheter-based interventions in an adult population in order to avoid surgical complications.
In addition to echocardiography, which is a primary investigative tool, an MRI or CT scan is a supportive, reliable and confirmatory diagnostic method for precise diagnosis and for planning management options of AAA. This is relevant in complex anatomic situations or severe obstruction because it provides better delineation of tissue and excellent spatial resolution.
In suitably selected patients, transcatheter recanalisation and stent implantation can be performed safely with success. A tortuous coarctation, long-segment involvement and arch hypoplasia may pose technical challenges. In this scenario, technical factors to be considered are types of catheter, balloons, stents, guidewires, and sheaths to be used and the speed or pressure of balloon inflation. In addition, antegrade or retrograde approach of perforation of the involved segment needs to be considered. Currently, covered stents have reduced the incidence of vessel rupture or aneurysm formation further due to their ability to stabilise the shape and integrity of the involved segment of the aorta13,14,15.
Simultaneous angiography above and below the obstruction (from two different access sites) should always be carried out to demonstrate clearly the space separating the aortic lumen on either side before recanalisation is attempted. It is difficult to predict what should be the safe distance between the contrast-filled aortic lumens on the two sides. If known, this prediction may help in selecting the right patients in order to avoid complications during recanalisation. In the presence of long segment involvement, a puncturing instrument could exit and re-enter the aorta, and therefore the balloon dilation of this track could have severe consequences.
The puncturing instrument used in this procedure may vary. The stiff end of a 0.014” coronary guidewire or a transseptal needle was frequently used in our series. Both instruments require mechanical force to puncture the site of obstruction and an appropriate needle curve that may require adjustment according to the anatomical situation. We should realise that the force applied is obviously not completely under control. The complications reported in this study occurred while using the stiff end of a coronary guidewire. A more controlled and less traumatic perforation may be obtained using RF energy to cross the obstruction5,9.
During the procedure, the placement of a gooseneck snare in the target vessel may help in directing the RF wire in the correct direction. The possibility of perforating highly elastic structures without excessive use of forward force and the ability to achieve better angles may be considered a possible advantage of RF over needle perforation.
In most of our patients the procedure was completed by deployment of covered stents, which are safer than bare metal stents. A theoretical risk of wall rupture and dissection still exists with the use of such stents; however, we did not experience such complications15,16,17. In our cohort, there were no long-term complications related to the stents used. The only need was for a second catheterisation procedure to re-dilate the existing stent. The types of existing stent were the NuMED CP (NuMED, Hopkinton, NY, USA) and Advanta stents.
Some technical difficulties may be encountered while negotiating a difficult narrowing of the aorta, especially when there is a tortuous segment or a figure of “3” sign. The possible complications in these cases could be difficulty in stent placement, migration of the stents, branch vessel involvement, perforations and a residual gradient. Therefore, careful planning is necessary before undertaking such cases and a surgical approach may be preferred, if warranted14,15,16,17,18. The aorto-oesophageal fistula is a little known but fatal complication of endovascular procedures. The possible mechanism could be a relatively thin and weaker wall of the aorta following angioplasty and projection of stents leading to erosion of the walls of the aorta and the oesophagus. The management of this entity involves endovascular intervention, aortic resection or conduit placement, which has been associated with high morbidity or mortality18.
A major point of the outcome is the persistence of systemic hypertension despite successful angioplasty. This outcome is not unexpected. Normalisation of blood pressure without medication after angioplasty for aortic coarctation occurs in 74 to 79% of adult patients19.
Limitations
This study is a multicentre, retrospective cohort involving five different centres around the world (Italy, Portugal, United Arab Emirates, Spain and Germany). The relatively small number of patients did not allow extensive multivariable modelling for data analysis. However, we were able to demonstrate that the percutaneous procedure in AAA cases had good outcomes with low mortality.
Conclusions
Percutaneous treatment of AAA using the three available standard catheter-based techniques is a safe, feasible and effective procedure as per our long-term follow-up outcome data. We suggest that the success of the procedure depends upon a carefully planned approach which should be limited to experienced centres. To our knowledge, this study represents the largest case series available in the literature so far. Although AAA remains a rare disease, we still need to assemble more data to validate percutaneous interventional success in those patients who require treatment, especially the adult population due to higher risks.
|
Impact on daily practice AAA is a very rare obstructive defect; it is an extreme form of coarctation of the aorta. The anatomic feature is the key to determining the feasibility of percutaneous interventions. So far, only limited data exist in the literature concerning the long-term follow-up following percutaneous recanalisation. In the current study, we have emphasised the fact that percutaneous treatment of AAA is a safe, feasible, reliable and effective procedure and has the prospect of favourable long-term outcomes. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

