Abstract
Aims: The 12-month results of RESOLUTE were favourable for the new Resolute stent. Two-year safety and efficacy results from RESOLUTE have been evaluated and are now reported.
Methods and results: RESOLUTE was a prospective, multicentre, non-randomised, single-arm, controlled trial of the Resolute stent in 139 participants with symptomatic ischaemic heart disease due to single de novo lesions in a native coronary artery. The 2-year rates of MACE (all-cause death, myocardial infarction, emergent cardiac bypass surgery, and target lesion revascularisation [TLR]), death, late stent thrombosis, target vessel revascularisation (TVR), and target vessel failure (TVF) were assessed. Clinical events included two MACE (one TLR; one non-cardiac death) occurring between year one and two resulting in cumulative 2-year TLR, TVR, and TVF rates of 1.4%, 1.4%, and 7.9%, respectively. One possible stent thrombosis event occurred in the first year after stent implantation however no late or very late ARC-defined definite and probable stent thromboses occurred through two years.
Conclusions: The 2-year data from RESOLUTE demonstrated no safety concerns including no late stent thrombosis or loss of effectiveness with the Resolute stent. The finding that few events occurred in year two is encouraging, yet requires verification in a larger population.
Abbreviations
ACC/AHA/SCAI: American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions
AR: Academic Research Consortium
BMS: bare metal stents
DAPT: dual antiplatelet therapy
DES: drug eluting stents
ITT: intent-to-treat
IVUS: intravascular ultrasound
MACE: major adverse cardiac event
MI: myocardial infarction
PCI: percutaneous coronary intervention
TIMI: Thrombolysis In Myocardial Infarction
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Drug eluting stents (DES) have consistently demonstrated improved outcomes in terms of clinical and angiographic restenosis as compared to bare metal stents (BMS)1-13. These improved outcomes have led to the wide adoption of DES in clinical practice. The 2005 European Society of Cardiology guidelines14 provided a class I recommendation for the use of DES in percutaneous coronary interventions (PCI) of de novo lesions in native vessels, and the most recent American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) guideline document similarly gives a Class I recommendation to the use of DES in patients who reflect the populations studied in clinical trials15.
Despite the benefits of DES over BMS, DES are still associated with a small risk of late and very late stent thrombosis. Regulatory approval of the first DES was based on randomised controlled trials assessing target vessel failure (TVF) or target vessel revascularisation (TVR) at nine months16,17. These trials generally demonstrated significantly lower rates of in-stent restenosis and target lesion revascularisation (TLR) during short-term follow-up. However, some studies with longer term follow-up revealed an increased risk of stent thrombosis after one year for patients receiving DES as compared to BMS13;18-20. The exact mechanism of very late stent thrombosis is not known, but it may be attributable to incomplete neointimal coverage, delayed or incomplete endothelialisation, late polymer reactions, strut fractures, positive remodelling with acquired stent malapposition, aneurysm formation, or new plaque rupture. These mechanisms may also be influenced by an insufficient duration of dual antiplatelet therapy (DAPT) and/or off-label use of DES in high-risk populations that have been underrepresented in clinical trials13;17-21. This historical context and the possibility that stent components may contribute to restenosis emphasises the importance of evaluating both short and long-term outcomes associated with emerging, novel stent technology.
The Resolute stent (Medtronic CardioVascular, Inc., Santa Rosa, CA, USA) is a next generation stent that employs a novel tri-polymer coating system. This system is designed to be biocompatible, thereby minimising the potential for hypersensitivity reactions that may contribute to late stent thrombosis. In addition, it was developed to extend zotarolimus drug elution to match potentially delayed arterial healing times common to lesions in high-risk patient subsets, such as those with diabetes, diffuse or multivessel disease, chronic renal failure, left main or ostial disease, or chronic total occlusions22.
The RESOLUTE trial was designed to evaluate the clinical safety and efficacy of the Resolute Coronary Stent System. The 12-month safety and efficacy measures from RESOLUTE have been reported23. Briefly, the Resolute stent demonstrated low in-stent late lumen loss, minimal neointimal hyperplastic in growth, low TLR, and acceptable TVF and major adverse cardiovascular events (MACE) rates at one year. Although the 12-month safety and efficacy endpoints for RESOLUTE were favourable for the next generation Resolute stent technology, it is critical to assess events at later time points to ensure durability of these results. The purpose of this analysis is to report the 2-year outcomes from the RESOLUTE trial.
Methods
Overview and study population
The RESOLUTE methodology and study procedures have been reported23. Briefly, RESOLUTE was a prospective, multicentre, non-randomised, single arm, controlled trial of the Resolute stent in 139 participants with symptomatic ischaemic heart disease. The study was conducted according to the Declaration of Helsinki, the protocol was approved by local ethics committees, and all participants provided voluntary, written informed consent.
Eligible participants included patients aged ≥18 years with symptomatic ischaemic heart disease due to single de novo stenotic lesions (>50% stenosis) in a native coronary artery with a reference vessel diameter ≥2.5 mm and ≤3.5 mm, a lesion length ≥14 mm and ≤27 mm, and a Thrombolysis In Myocardial Infarction (TIMI) flow grade ≥2. Patients with a recent (<72 hours) myocardial infarction (MI), prior stent placement within 30 days, or any general contraindication to the revascularisation procedure or routine DAPT were not eligible for participation. Patient follow-up was planned at six months and 1, 2, 3, 4, and 5 years post-procedure. Participants were enrolled at 12 centres in Australia and New Zealand between December 6, 2005 and October 30, 2006.
Study endpoints
The primary endpoint of the RESOLUTE study was in-stent late lumen loss at nine months post-procedure as measured by quantitative coronary angiography23. Secondary endpoints included MACE (defined as death, MI [Q wave and non-Q wave], emergent coronary artery bypass graft [CABG] surgery, or TLR [repeat PCI or CABG]) at 30 days, and 4, 6, 9, and 12 months post-procedure; acute device, lesion, and procedural success; angiographic endpoints at four or nine months; and TVF and TLR at nine months. For the purpose of this analysis, clinical outcomes (MACE, death, vascular complications, late stent thrombosis, stroke, TLR, TVR, and TVF) were reported for the 2-year follow-up.
Study procedures
All participants received aspirin ≥75 mg/day at least 24 hours prior to the procedure and a clopidogrel loading dose of ≥300 mg within 24 hours prior to the procedure, followed by aspirin ≥75 mg/day indefinitely and clopidogrel 75 mg/day for a minimum of six months. Heparin was administered to maintain an activated clotting time ≥250 seconds or between 200 seconds and 250 seconds if a glycoprotein IIb/IIIa receptor inhibitor was administered. Percutaneous coronary interventions (PCI) were performed in accordance with the local hospital’s standards. Predilation was mandatory and undertaken with a balloon length shorter than the stent. Post-dilation, if required, was also carried out with a balloon length less than or equal to that of the stent. Resolute stents were available in diameters ranging from 2.5 mm to 3.5 mm and in lengths from 8 mm to 30 mm.
Device overview
The Resolute stent was designed with a novel proprietary biomimetic polymer drug carrier. The Resolute stent uses the same Driver (Medtronic) bare metal stent platform and contains the same concentration of zotarolimus (1.6 mcg/mm2) as the Endeavor (Medtronic) stent. The strut thickness is 0.0036 inches (9.14 µm)24. The new polymer coating of the Resolute stent is based on the BioLinx polymer system and consists of a unique blend of three different polymers, a hydrophilic C19 polymer, a water soluble polyvinyl pyrrolidinone (PVP) polymer, and a hydrophobic C10 polymer22,25. The average drug/polymer coating thickness is 4.1 µm.
This combination of polymers acts to mimic human biochemistry, and it provides a hydrophilic platform that minimises the in vivo inflammatory responses25,26. These polymers were also designed to provide extended drug release. PVP provides an initial drug burst, whereas the more hydrophobic C10 polymer allows zotarolimus to be released over time with complete elution by 180 days22,25.
Data management
At two years post-procedure, participants were contacted by telephone to determine the occurrence of MACE defined as death, MI (Q wave and non-Q wave), emergent cardiac bypass surgery, or target lesion revascularisation (repeat PTCA or CABG), document angina class and document dual antiplatelet/anticoagulation therapies. The Harvard Clinical Research Institute (HCRI, Boston, MA, USA) independent clinical events committee followed up on all reported events to obtain source documents for review and adjudication of each event.
Statistical considerations
The analyses were conducted according to the intent-to-treat (ITT) principle. For the 2-year follow-up, all participants who were enrolled in the study were included in the analysis. Clinical outcome variables were reported as rates. In addition, Kaplan-Meier estimates of the proportion of participants free of MACE, TLR, TVR, TVR, and stent thrombosis at two years were calculated. In addition, comparisons between groups were presented with P-values generated with Fisher exact test for binary endpoints. All statistical analyses were performed using SAS for Windows (version 8.2 or higher, SAS Institute, Cary, NC, USA).
Results
A total of 139 participants (140 lesions) were enrolled in RESOLUTE. After enrolment of the first 130 participants, further enrolment was limited to subjects who would also agree to participate in a pharmacokinetic substudy. An additional nine participants were enrolled in this substudy, yielding a total study cohort of 139 participants. Two-year follow-up was complete in 100% of participants. No participants were lost to follow-up.
Baseline characteristics of the overall study population have been reported23. The mean age of patients was 61 years and the majority were men (76.3%). Almost one-half of the participants had a previous MI (46.4%), and 18.7% had a prior PCI. The mean lesion length was 15.61±6.13 mm, and the mean reference vessel diameter was 2.81±0.40 mm. The mean percent diameter stenosis decreased from 70.30±11.37% pre-intervention to 3.36±8.54% post-intervention. RESOLUTE included some high-risk patient groups, including diabetes mellitus (17.3%), lesion length >20 mm (23.7%), and overlapping stents (1.4%).
The safety measures up to 720 days are shown in Table 1.
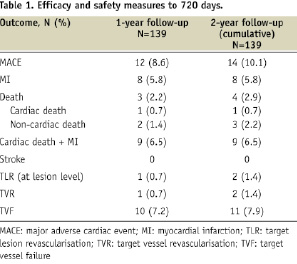
Clinical events from the 1-year follow-up are also shown for comparison. There were two MACE events (one TLR at 573 days for symptomatic 52% in-stent restenosis per QCA and one non-cardiac death at 665 days due to adenocarcinoma of the lower oesophagus with liver metastases) resulting in 2-year TLR, TVR, and TVF rates of 1.4%, 1.4%, and 7.9%, respectively (Figure 1).
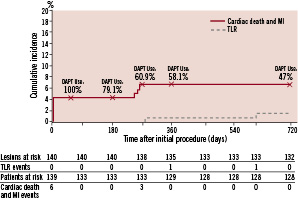
Figure 1. Cumulative incidence of TLR and cardiac death and MI through two years, and percent DAPT use at 30 days, and 6, 9, 12, and 24 months.
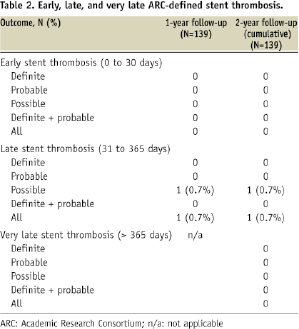
There were six periprocedural non-Q wave MIs that account for the large discrepancy between TVF and TLR/TVR rates. These events were described in the one year report23. There were no additional MIs between year one and year two. The 2-year Kaplan-Meier estimate of cumulative incidence of TLR was 1.5%. No stent thrombosis events occurred between one and two years (Table 2). There were no protocol-defined stent thrombosis events through two years.
The proportion of participants who were maintained on DAPT during follow-up is shown in Figure 1. At two years, the majority of participants were still taking aspirin (94.8%), 50% were taking clopidogrel, and 47.1% were taking dual antiplatelet therapy with aspirin and clopidogrel.
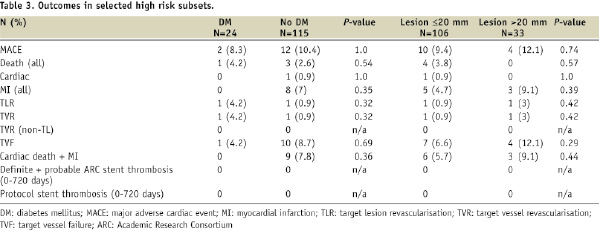
Cumulative 2-year safety events were also evaluated in select high risk subgroups as a hypothesis generating analysis. Patients with diabetes were slightly older (64.1±10.2 vs. 60.0±9.9 years) and had a higher incidence of hypertension as compared to non-diabetic patients. The number of events in each subgroup was low, limiting the ability to detect differences between subgroups, but the rate of MACE, TLR, TVR, and TVF appeared to be similar in participants with diabetes as compared to those without diabetes, and there were no protocol-defined stent thrombosis events. No differences in clinical events were detected among patients with long lesions (>20 mm) as compared to those with shorter lesions (≤20 mm) (Table 3).
Late incomplete stent apposition was an endpoint of interest in the RESOLUTE 1-year follow-up analysis. Intravascular ultrasound (IVUS) performed at nine months revealed late acquired incomplete stent appositions in six participants. No adverse events occurred in any of these six patients during follow-up between one and two years.
Discussion
This analysis of 2-year follow-up from the RESOLUTE trial revealed encouraging results in terms of the long-term safety and durable efficacy of the next generation Resolute stent. In RESOLUTE, no evidence of very late stent thrombosis or related adverse clinical outcomes was detected. Only two MACE occurred between one and two years, which included one non-cardiac death and one TLR. These additional events resulted in 2-year cumulative event rates of MACE (10.1%), all-cause death (2.9%), TLR (1.4%), TVR (1.4%), and TVF (7.9%). The TLR event involved direct stenting of a 52% in-stent restenosis in a patient with presumed angina. The non-cardiac death occurred in a patient diagnosed with metastatic oesophageal adenocarcinoma. Importantly, there were no cases of stent thrombosis between one and two years. Cumulative TLR-free survival was 98.5%, and stent thrombosis free survival was 100%. As previously reported, the 9-month IVUS detected six cases of late acquired incomplete stent apposition, however only one case resulted in a clinical event (TLR at 280 days) and no further adverse events occurred out to two years.
These results are encouraging, but longer term follow-up of a larger population will be needed to assess if the safety profile of the Resolute stent will be similar to the Endeavor stent, which has demonstrated extremely low rates of stent thrombosis beyond one year27. Both devices release zotarolimus, but the Resolute stent has been developed with a novel, hydrophilic polymer that optimises both biocompatibility and drug elution22. This polymer has been tested in a porcine model, and it demonstrated no inflammatory response. Inflammation scores were similar between polymer-coated and bare metal stents in this animal model, and they were also similar to inflammatory scores with the Endeavor stent22. In addition, the zotarolimus-polymer complex has been designed to release an initial burst of drug over the first 48 hours. A slower, sustained release is then achieved that results in effective arterial wall drug concentrations up to 180 days post-implantation and inhibition of neointimal proliferation for up to 90 days, characteristics which may provide longer protection against restenosis22.
Proposed hypotheses to explain the very late stent thromboses that have been observed with earlier stent systems include incomplete neointimal coverage, delayed or incomplete endothelialisation, late polymer reactions, strut fractures, positive remodelling with stent malapposition, aneurysm formation, or new plaque rupture, which may be enhanced by an insufficient duration of dual antiplatelet therapy (DAPT) and/or off-label use of DES in high-risk populations13,17,21. Many of these proposed mechanisms relate to features of the stent itself, and it is possible that the polymer used in the Resolute stent system does not result in these adverse responses.
The RESOLUTE study included some patients with higher-risk characteristics. The use of DES in higher-risk patients, such as those with diabetes, long lesions, or overlapping stents who have generally been underrepresented in pivotal trials, has been proposed as a potential reason for the increased risk of late stent thrombosis16,17. In the RESOLUTE 2-year follow-up data, longer-term safety appeared to be maintained even in the higher-risk subsets of patients with diabetes and long lesions, although the number of patients and events within these subgroups were small and definitive conclusions cannot be drawn from this subgroup analysis. This observation further supports the hypothesis that the Resolute next generation polymer may provide more comprehensive protection against late stent thrombosis.
Another contributing factor to the favourable safety outcomes may have been the relatively high use of DAPT. At two years, nearly half of the participants continued to receive both aspirin and clopidogrel. Between years one and two, an additional 11% of participants discontinued DAPT. Because of the small number of clinical events, it is not possible to assess the contribution of DAPT discontinuation on outcomes in this analysis. The relative importance of long-term DAPT (beyond 1-year) in preventing late stent thrombosis has not been determined, but it is possible that the sustained use of DAPT influenced the low occurrence of clinical events in RESOLUTE.
These findings should be evaluated in the context of several study limitations. First, RESOLUTE was a non-randomised, single-arm study. Thus, observations can be drawn from these data, but definitive conclusions regarding the efficacy and safety of the Resolute stent as compared to the Endeavor or other DES cannot be made. The numbers of subjects within the high-risk subgroups were small, which limits the interpretation of these data. However, the subgroup analyses were performed as hypothesis generating analyses, and additional studies may be designed to evaluate the Resolute stent in these populations.
In summary, the 2-year follow up data from the RESOLUTE trial demonstrated no evidence of longer-term safety concerns with the Resolute stent. The incidence of major adverse clinical events was low, and few events occurred between years one and two. The technology employed in the Resolute stent system appears to have a sustained safety profile between years one and two that is consistent with the safety findings from earlier studies evaluating this stent technology. Data are needed from larger randomised studies, such as the RESOLUTE All-Comers study (www.clinicaltrials.gov, NCT00617084) to fully clarify the mechanisms of late stent thrombosis and effective methods for reducing this risk.
Acknowledgements
The authors thank Wendy Gattis Stough, PharmD and Colleen Gilbert, PharmD from CommGeniX, LLC for assistance in the preparation of this manuscript.
The RESOLUTE Stent Trial Investigators: Australia: R. Hendriks, G. Tulloch, D. Greenwell, Fremantle Hospital, Fremantle; M. Horrigan, L. Brown, Austin Health Medical Centre, Melbourne; I. Meredith, MA. Austin, J. Plunkett, Monash Medical Centre, Melbourne; D. Muller, E. O’Dea, St. Vincent’s Hospital, Sydney; D. Walters, T. Christensen, R. Pincus, Prince Charles Hospital, Brisbane; R. Whitbourn, C. Peeler, J. Wilson, St. Vincent’s Hospital, Melbourne; S. Worthley, D. Yudkin, A. Roach, V. Maxwell, Royal Adelaide Hospital, Adelaide.
New Zealand: P. Matsis, D. Middleditch, Wellington Hospital, Wellington; D. McClean, M. Hume, Christchurch Hospital, Christchurch; J. Ormiston, A. Fraser, Mercy Hospital, Auckland; J. Ormiston, S. Simpson-Plaumann, Auckland City Hospital, Auckland; G. Wilkins, M. Blok, Dunedin Hospital, Dunedin.

