Introduction
Anecdotal series have suggested little correlation between the lipid content of the coronary arteries, the maximal Lipid Core Burden Index within any 4 mm segment (maxLCBI4mm) as measured by near infrared spectroscopy-intravascular ultrasound (NIRS-IVUS) or its change over time, and the serum-measured low-density lipoprotein cholesterol (LDL-C), but they have shown modest association with high-density lipoprotein cholesterol (HDL-C)12. Whether these relationships hold in a larger data set remains unknown. The Lipid Rich Plaque (LRP) study (ClinicalTrials.gov: NCT02033694) showed that lipid-rich plaques (maxLCBI4mm >400) were associated with a greater incidence of non-culprit major adverse cardiovascular events3. The current subanalysis reviews the correlation between maxLCBI4mm and both the LDL-C and HDL-C among the patients enrolled in the LRP study.
Methods
The LRP study enrolled 1,563 patients, of whom 984 had both evaluable maxLCBI4mm and serum cholesterol data. In brief, patients presenting to the cardiac catheterisation laboratory with possible ad hoc percutaneous coronary intervention (PCI) were enrolled and underwent NIRS-IVUS to a minimum of 50 mm of non-stented vessels. All patients provided informed consent before catheterisation, and ethics approval was obtained from all participating centres3. NIRS-IVUS analyses were done offline by an independent core laboratory (MedStar Cardiovascular Research Network, QIVUS version 3.0.16.0; Medis Medical Imaging Systems). Statistical comparisons (performed in SAS 9.4) were done with the chi-squared test or Fisher’s exact test for categorical data, the Student’s t-test for continuous data, and Pearson’s correlation coefficient to estimate the correlation.
Results
The baseline characteristics of the overall cohort in the subanalysis were similar to the main study, with a mean age of 64.0 years, 71.1% male, 37.8% with diabetes mellitus, 21.9% current smokers, 25.0% with prior myocardial infarction, and 48.3% with prior PCI3. Overall, 78.2% of the population was on a statin at baseline, predominantly atorvastatin (41.1%), followed by simvastatin (17.0%), rosuvastatin (14.4%), pravastatin (5%), and others. The mean LDL-C for patients presenting on versus off statin therapy was 84.6±37.0 mg/dL vs 114.8±42.8 mg/dL (p<0.001), while the HDL-C was 44.2±13.6 mg/dL vs 45.3±18.5 mg/dL (p=0.405), the total cholesterol was 155.2±41.6 mg/dL vs 187.0±47.8 mg/dL (p<0.001), and the triglycerides were 141.9±89.7 mg/dL vs 157.9±124.9 mg/dL (p=0.080). The correlations of maxLCBI4mm vs LDL-C or HDL-C show a lack of any correlation with baseline LDL in patients taking statins, but a marginal correlation in statin-naïve patients (r=0.028, p=0.438; r=0.134, p=0.050, respectively). Without the dichotomisation of statin therapy upon enrolment, the overall correlation was r=0.194, p=0.041. There were no correlations with HDL-C (r=-0.042, p=0.191) (Figure 1).
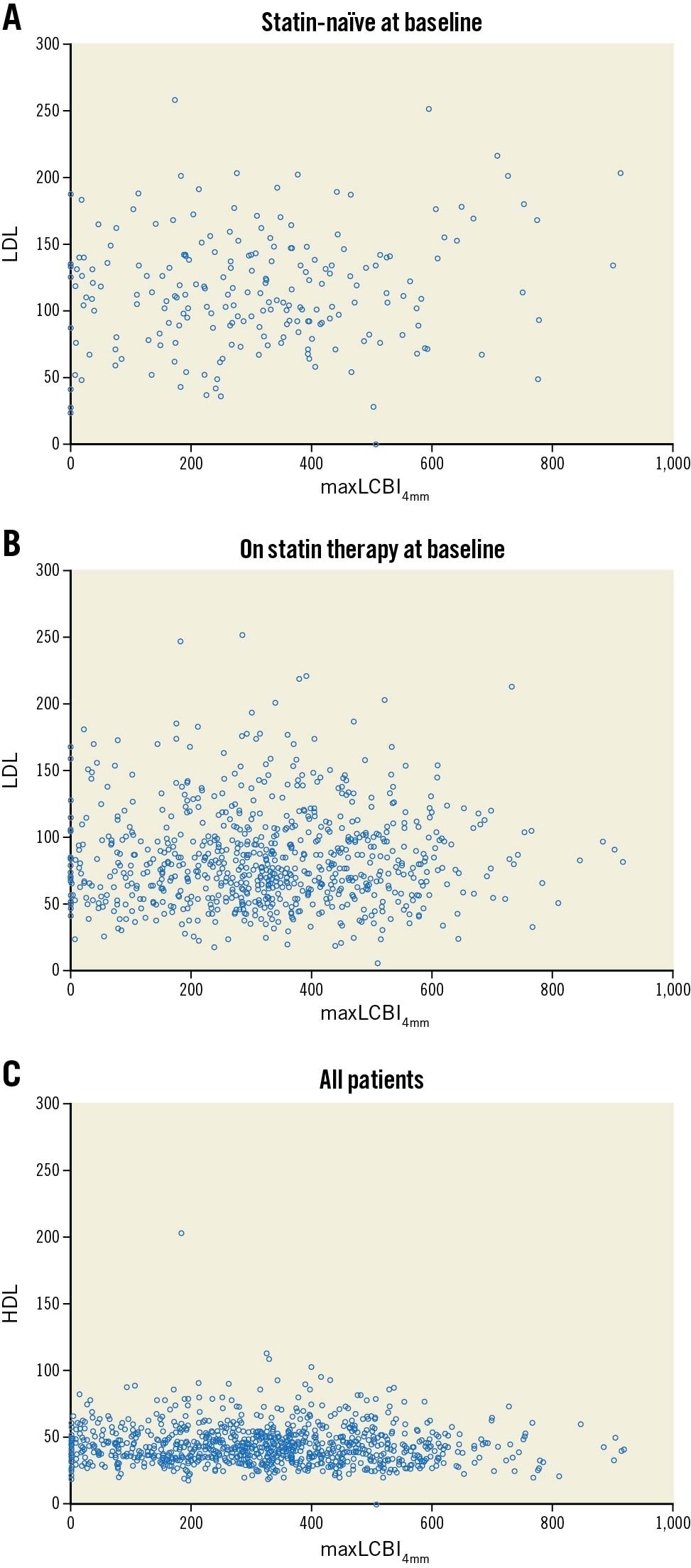
Figure 1. Correlations between maxLCBI4mm and LDL-C and HDL-C. A. Statin-naïve patients at baseline maxLCBI4mm vs LDL cholesterol; B. Patients taking statins at baseline, maxLCBI4mm vs LDL cholesterol; C. Overall baseline maxLCBI4mm vs HDL cholesterol. HDL: high-density lipoprotein; LCBI: lipid core burden index; LDL: low-density lipoprotein; maxLCBI4mm: maximum lipid core burden index in a 4 mm segment.
Discussion
These data corroborate the findings by Dobrolinska et al of no correlations between LDL-C and maxLCBI4mm (r=0.105, p=0.549), although in contrast to our data, Dobrolinska et al reported a weak correlation with HDL-C (r=-0.035, p=0.007)1. Another recent analysis of 49 patients undergoing serial NIRS demonstrated that a change in HDL-C was correlated with a change in arterial lipidic content, with a negative correlation at 13 months (p=0.004)2. Additionally, Takata et al presented a series of 47 patients with repeated NIRS measurements where high HDL-medicated cholesterol efflux capacity via the adenosine triphosphate (ATP)-binding cassette transporter G1 pathway was associated with a regression of maxLCBI4mm4. Conversely, two serial NIRS-IVUS treatment studies, one prospective and one retrospective, demonstrated that a reduction in LDL-C with either high-intensity rosuvastatin (40 mg) or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition was accompanied by a reduction in maxLCBI4mm5. If these data are representative of the true relationship between LDL-C and the lipidic content of coronary arteries, plaque-modification strategies specifically to alter this lipidic content should be a focus going forward.
Limitations
The present post hoc analysis has a few limitations. First, the guideline-recommended LDL-C management appears to be suboptimal for those patients on statin therapies; additionally, these data were collected prior to the introduction of PCSK-9 inhibitors. Second, the intent of the LRP study was to evaluate images of untreated arteries; therefore, the lipidic content of the culprit lesions was not a part of this dataset. Last, serial cholesterol panel data or imaging data were not available.
Conclusions
In conclusion, in the LRP study, we could not show any relationship between NIRS-IVUS-measured maxLCBI4mm and serum-measured LDL-C in statin-treated patients, while a weak correlation did exist for statin-naïve patients; and we could not show any relationship between NIRS-IVUS-measured maxLCBI4mm and serum-measured HDL-C. Further investigation is needed to understand the relationship between the free-floating cholesterol biomarkers and the arterial lipids.
Conflict of interest statement
G. Mintz reports honoraria from Boston Scientific, Philips, Medtronic, and Abiomed. C. Di Mario reports institutional grants from Amgen, Behring, Chiesi, Daiichi Sanyo, Edwards Lifesciences, Medtronic and Shockwave Medical and received speakers’ fees from Philips. H. Garcia reports institutional grants from Medtronic, Biotronik, Neovasc, Boston Scientific, Abbott, Shockwave, Chiesi and Phillips. R. Waksman serves on the Advisory Boards of Abbott Vascular, Boston Scientific, Medtronic, Philips IGT, Pi-Cardia Ltd.; is a consultant for Abbott Vascular, Biotronik, Boston Scientific, Cordis, Medtronic, Philips IGT, Pi-Cardia Ltd., Swiss Interventional Systems/SIS Medical AG, Transmural Systems Inc. and Venous MedTech; has received grant support from AstraZeneca, Biotronik, Boston Scientific, Chiesi, Medtronic and Philips IGT; is a member of the Speakers Bureau of AstraZeneca and is an investor in MedAlliance and Transmural Systems Inc. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

