
Abstract
Aims: Statins are highly effective in reducing major adverse clinical events, but the direct effects on coronary plaque composition remain debatable. Our aim was to mechanistically evaluate the treatment effect of high-intensity statin therapy on compositional coronary plaque changes.
Methods and results: The third Integrated Biomarker and Imaging Study (IBIS-3) was a prospective, investigator-initiated, single-centre study. Serial radiofrequency intravascular ultrasound (RF-IVUS) measurements of a predefined non-stenotic segment in a non-culprit coronary artery were performed to evaluate the effect of rosuvastatin (intended dose: 40 mg daily) on necrotic core (NC) volume in patients with stable angina or acute coronary syndrome. Changes in lipid core burden index (LCBI) were evaluated through serial near-infrared spectroscopy (NIRS) imaging in a subset. Serial RF-IVUS (and NIRS) data of a median segment of 41 mm (interquartile range: 32 to 49 mm) were complete in 164 (103) patients. Follow-up measurements were performed at six and 12 months in 30 (26) and 134 (77) patients, respectively. Mean levels of low-density lipoprotein cholesterol decreased by 30%, from 2.49 mmol/l to 1.73 mmol/l at the end of follow-up. High-dose rosuvastatin therapy resulted in a non-significant change of –1.4 mm3 (95% CI: –3.0, 0.1) in NC volume during follow-up (p=0.074). The change in NC percentage of total plaque volume was –1.4% (95% CI: –2.4 to –0.4; p=0.006). A neutral effect was also observed on LCBI. Indications of significant regression of NC volume and LCBI in the highest baseline quartiles were observed, which should cautiously be regarded as hypothesis-generating.
Conclusions: High-intensity rosuvastatin therapy during one year resulted in a neutral effect on NC and LCBI within non-stenotic, non-culprit coronary segments with a relatively low atheroma burden. This study has been registered in The Netherlands Trial Register (NTR) nr. 2872.
Abbreviations
ACS: acute coronary syndrome
CAG: coronary angiography
IBIS: Integrated Biomarker and Imaging Study
LCBI: lipid core burden index
LDL-c: low-density lipoprotein cholesterol
NC: necrotic core
NIRS: near-infrared spectroscopy
PCI: percutaneous coronary intervention
RF-IVUS: radiofrequency intravascular ultrasonography
SAP: stable angina pectoris
Introduction
The presence of coronary plaque phenotypes with large necrotic core (NC) volumes is associated with a high incidence of major adverse cardiac events1-3. In the second Integrated Biomarker and Imaging Study (IBIS-2), the lipoprotein-associated phospholipase A2 (Lp-PLA2) inhibitor darapladib – added to statins – halted coronary NC volume progression4. We now report IBIS-3, which evaluated high-dose rosuvastatin to reduce coronary NC volume, assessed by radiofrequency intravascular ultrasound (RF-IVUS), and intracoronary cholesterol accumulation, assessed by near-infrared spectroscopy (NIRS)5.
Methods
The IBIS-3 study details have been published elsewhere5. Briefly, patients undergoing coronary angiography (CAG) or percutaneous coronary intervention (PCI) were treated with a high dose (40 mg daily) of rosuvastatin for 12 months. Near the completion of the study, the protocol was amended to enable a treatment duration of six months. IBIS-3 was approved by the Medical Ethics Committee of the Erasmus MC. Written informed consent was obtained from all participants.
Subsequent to the index CAG/PCI, RF-IVUS was performed in a non-culprit coronary segment with the Eagle Eye® catheter (Volcano Corp., San Diego, CA, USA) and NIRS with the Infraredx system (Infraredx, Burlington, MA, USA), at a pullback speed of 0.5 mm/sec. Initially, the NIRS system was non-CE-marked and several patients refused to provide consent for its use. Intracoronary imaging was repeated at the end of the scheduled rosuvastatin treatment period. RF-IVUS and NIRS images were analysed offline by an independent core laboratory (Cardialysis, Rotterdam, The Netherlands).
The primary endpoint was the change in NC volume. Secondary endpoints included the change in NC percentage, and the change in NIRS-derived lipid core burden index (LCBI) for the entire region of interest (ROI), and the 10 mm and 4 mm segments with the highest LCBI, the LCBImax10 mm and LCBImax4 mm, respectively.
We aimed to enrol 300 patients. Assuming an attrition rate of 15%, the sample size was determined at 350 patients5. The actual attrition rate appeared to be approximately 30% (Figure 1). We therefore decided to terminate patient enrolment in June 2013.
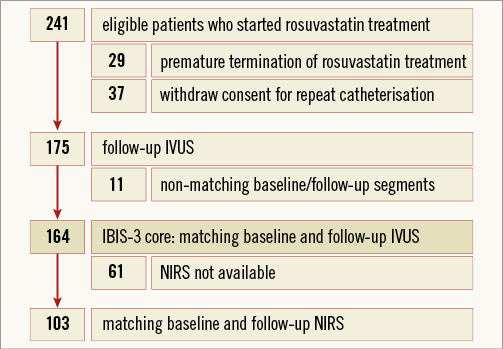
Figure 1. Study flow chart describing inclusion, attrition and the final IBIS-3 core of 164 patients with matching baseline and follow-up RF-IVUS. IVUS: radiofrequency intravascular ultrasound; NIRS: near-infrared spectroscopy.
STATISTICAL ANALYSIS
The study design paper specified that treatment effects would be tested with paired Student’s t-tests5. However, because the study endpoints had non-normal distributions, we decided to perform non-parametric statistics instead. Furthermore, we decided to square our data analysis methods with the IBIS-4 study, including the use of linear mixed models and regression6. We report changes in serum cholesterol levels and study endpoints as follow-up minus baseline values, and negative values indicate a decrease over time. All statistical tests were two-sided, and a p-value <0.05 was considered statistically significant.
Results
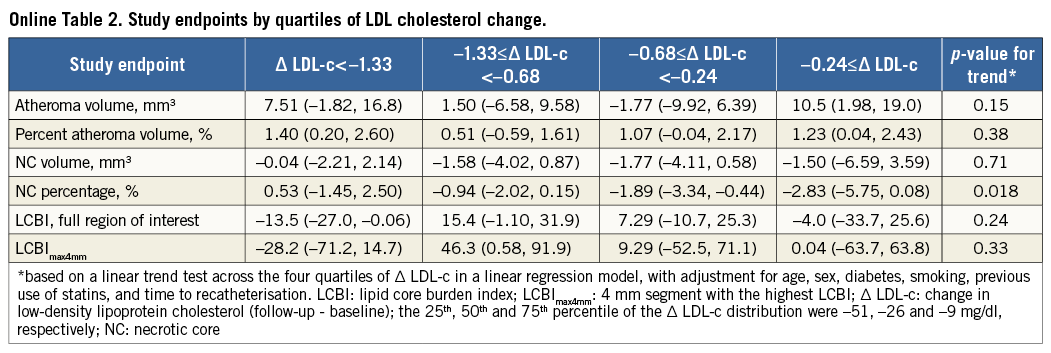
Serial RF-IVUS was available in 164 patients, including 103 with serial NIRS (Figure 1). Table 1 shows baseline characteristics. Rosuvastatin was taken during a median of 372 (interquartile range: 357 to 395) days, with 90.9% of the patients being titrated to the maximum dose. At the time of the recatheterisation, 92% of patients were on rosuvastatin 20-40 mg (Online Table 1).
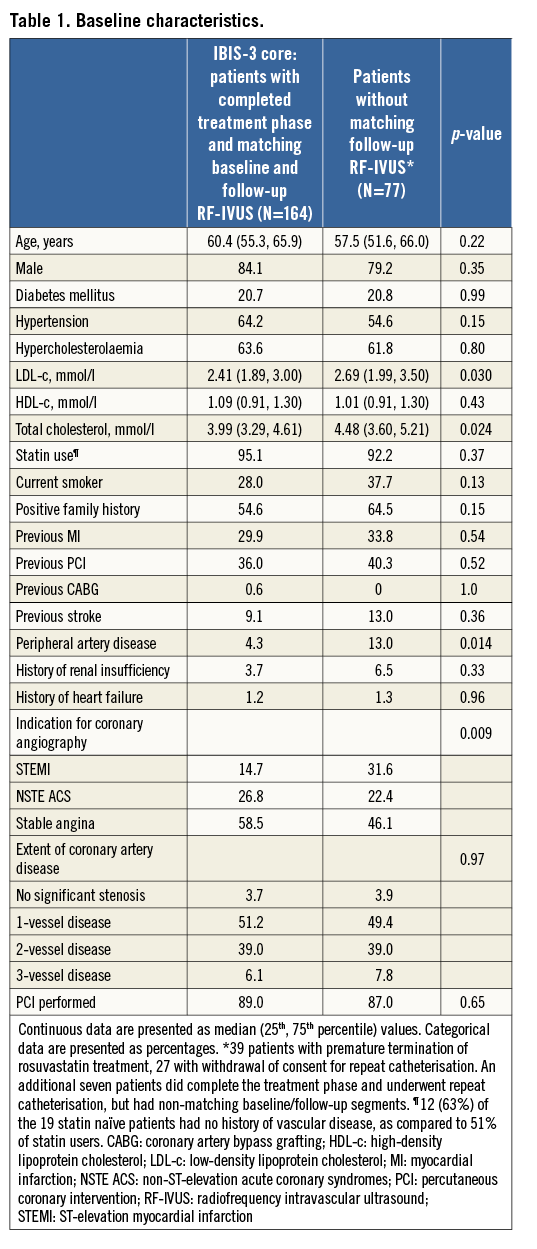
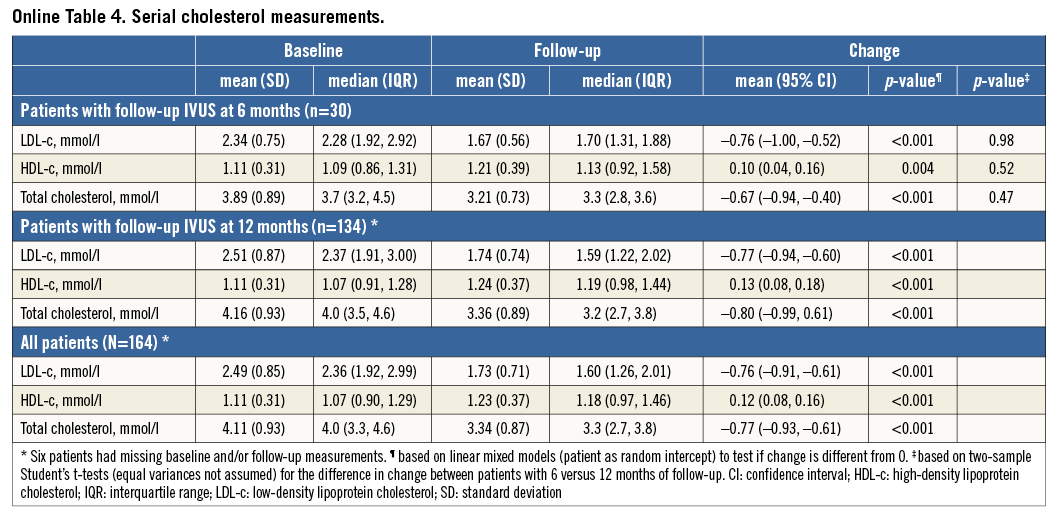
Mean LDL-c decreased by 30%, from 2.49 to 1.73 mmol/l, and HDL-c increased by 11%, from 1.11 to 1.23 mmol/l (Table 2, Online Figure 1).
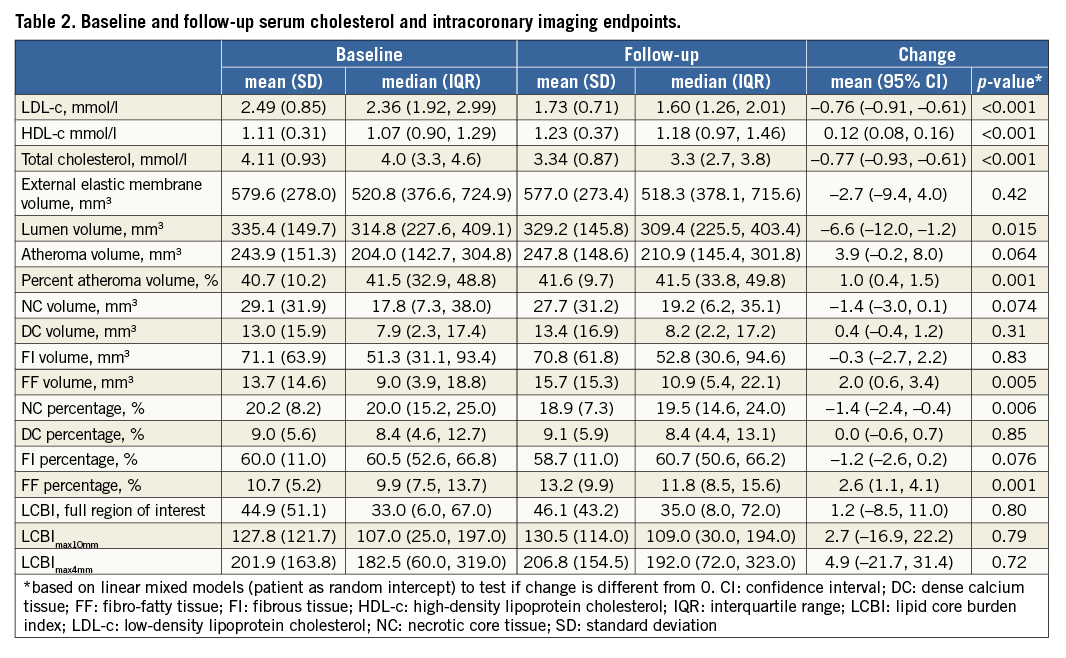
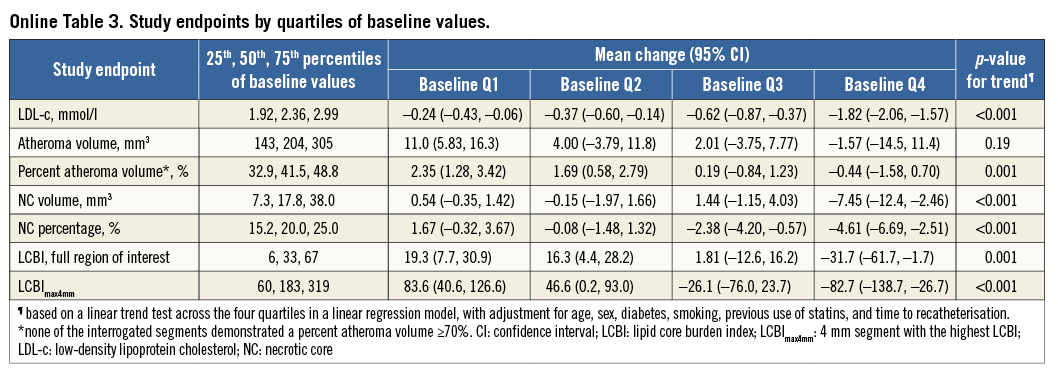
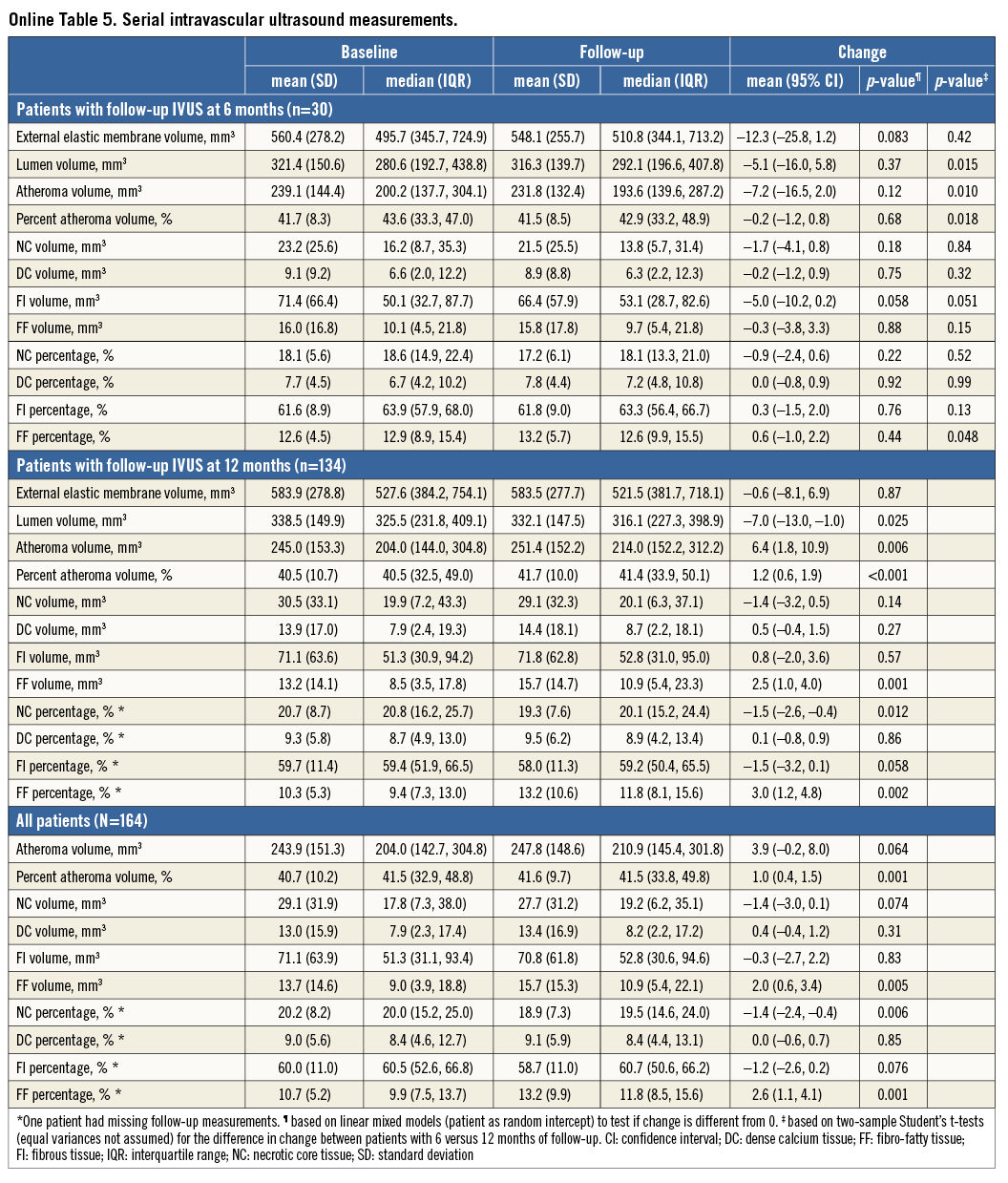
A change of –1.4 mm3 (95% confidence interval [CI]: –3.0 to 0.1) in NC volume was observed (Table 2, Figure 2). The change in NC percentage of total plaque volume was –1.4% (95% CI: –2.4 to –0.4). The latter finding should be interpreted in conjunction with a modest, but significant rise in percent atheroma volume (PAV). The change in serum LDL-c levels did not correlate with the change in coronary plaque characteristics (Online Table 2, Online Figure 2). Regression of NC volume was observed in patients within the highest baseline quartile (Online Table 3, Figure 2).
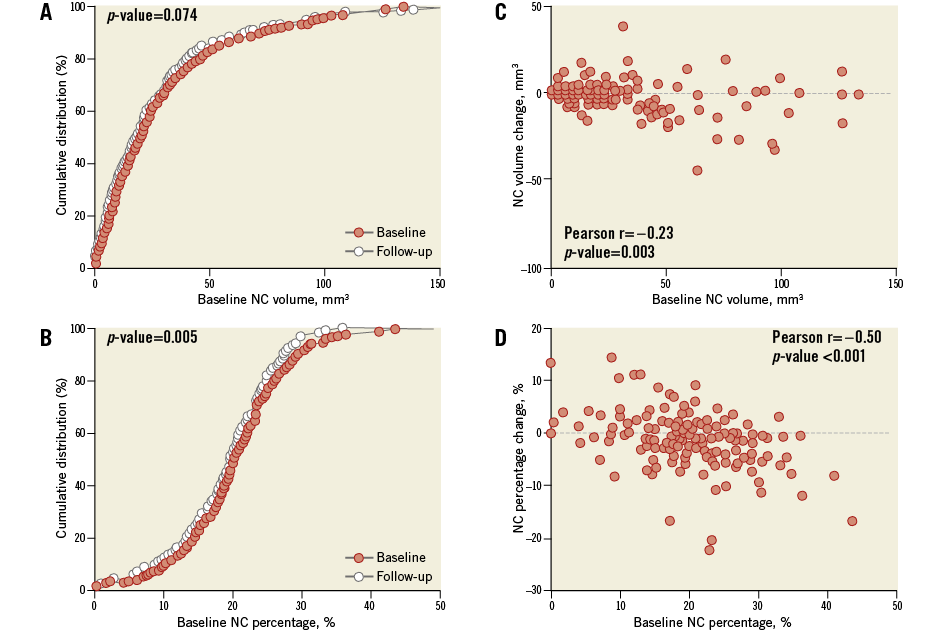
Figure 2. Necrotic core volume and percentage at baseline and follow-up. High-intensity rosuvastatin therapy led to a neutral effect on NC volume (A) and a significant decrease in NC percentage (B). The highest reductions were observed in those patients with relatively high necrotic core burden at baseline. Panel C depicts the change of NC volume under high-intensity rosuvastatin therapy against the baseline NC volume. Panel D illustrates the same for NC percentage. NC: necrotic core
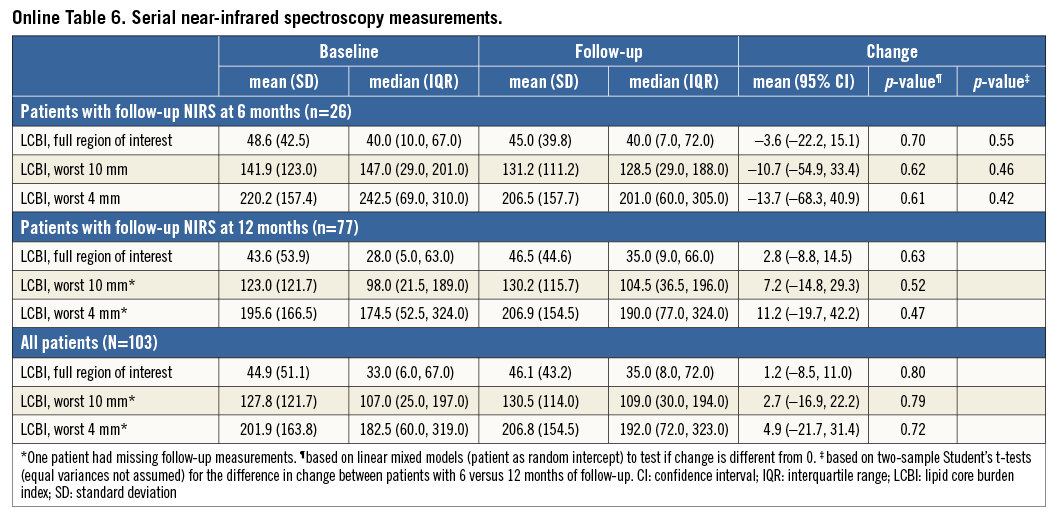
Within the 103 patients with repeat NIRS, changes in LCBI were non-significant (Table 2, Figure 3). LCBI regression might be pronounced in the highest baseline quartile (Online Table 3, Figure 3). There was no correlation between LDL-c change and LCBI change (Online Table 2, Online Figure 2). LCBI change was similar in statin-naïve patients and prior statin users.
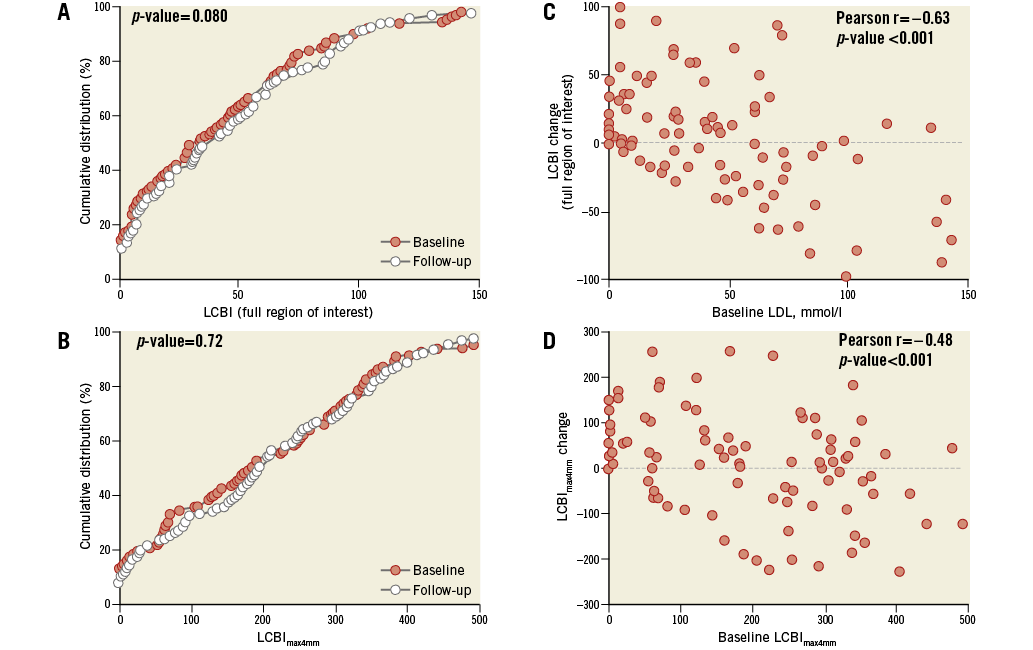
Figure 3. LCBI at baseline and follow-up. The effect of high-intensity rosuvastatin therapy on LCBI of the full region of interest (A) and the LCBImax4mm (B). Panels C and D indicate that the degree of LCBI regression might be dependent on the baseline LCBI. LCBI: lipid core burden index; LCBImax4mm: the 4 mm segment with the highest LCBI
All effects were similar in patients with repeat imaging at six and 12 months (Online Table 4-Online Table 6).
Discussion
High-intensity rosuvastatin therapy resulted in a neutral effect on NC and LCBI within non-stenotic coronary segments with a relatively low baseline atheroma burden. IBIS-2 showed a stabilisation of NC volume by darapladib, with 91% of patients on statin therapy4. IBIS-3 suggests that NC stabilisation might be possible with a potent statin alone.
Our findings concur with a meta-analysis of 17 studies involving 2,171 patients on at least six different statins, which showed that longer-duration and higher-intensity statin therapy may result in plaque volume regression, but not in a significant NC reduction7. Lack of change in NC burden after high-intensity statin therapy was also observed in SATURN8 and in IBIS-4, which studied STEMI patients6.
The YELLOW trial demonstrated a significant LCBI reduction in 44 patients after six to eight weeks of high-intensity rosuvastatin therapy9. In the comparator group of 43 patients, who were kept on their “regular” statin, the LCBI remained unchanged. However, YELLOW evaluated the effect of rosuvastatin on untreated obstructive coronary lesions with a fractional flow reserve <0.8. In contrast, we studied non-flow-limiting coronary segments with a low median LCBI of 33 (versus 95-132 in YELLOW). As a consequence, high-intensity statin therapy in IBIS-3 only had a limited substrate with respect to regression of LCBI. Still, our observation of a significant LCBI reduction in patients with high baseline values might be relevant, since those patients in particular are at increased risk of adverse cardiac events10.
The fact that changes in NC and LCBI were not correlated to changes in serum LDL-c levels may support the abundance of data on the pleiotropic effects of statins that are not directly related to serum lipid levels11. We only studied the effect of rosuvastatin on plaque composition in relation to its effect on LDL-c. However, recent studies suggest that LDL-c will not be atherogenic until it becomes oxidised in the arterial wall11.
IBIS-3 was an uncontrolled, observational study, similar to IBIS-4 and ASTEROID6,12. A disadvantage of such an approach is that true treatment effects cannot be distinguished from “regression to the mean”. In our study, the most pronounced regression of plaque components occurred within the highest baseline quartiles, which might be an expected and logical consequence of a real treatment effect. On the other hand, the simultaneous increase in most plaque parameters that was observed in the lowest baseline quartiles is suggestive of at least a component of regression to the mean.
IBIS-3 was designed to be embedded in our routine clinical practice, which we consider important for external validity. Consequently, however, the IBIS-3 patients were somewhat older and had more comorbidities than those observed in similar studies with repeat imaging12,13, which may explain their higher than expected drop-out rate. We enrolled 164 of 300 planned patients with repeat IVUS. The observed 1.4 mm3 NC reduction was smaller than anticipated5, but the standard deviation was also smaller (10.0 versus 13.9 mm3). Consequently, the power of IBIS-3 was still high enough (90%) to declare the anticipated 2.5 mm3 NC reduction statistically significant, but too small (50%) with regard to the observed effect.
Conclusion
The IBIS-3 study, a prospective, mechanistic, single-arm, open-label study designed to evaluate the treatment effect of high-intensity rosuvastatin therapy, demonstrated a neutral effect on NC volume in a non-culprit coronary artery segment without significant luminal narrowing. Indications of regression of NC percentage and NC volume and LCBI in the highest baseline quartiles should only be cautiously regarded as hypothesis-generating.
| Impact on daily practice IBIS-3 was designed to elucidate the already proven effectiveness of rosuvastatin therapy on cholesterol and clinical event reduction from a mechanistic approach by evaluating its effects on relevant coronary plaque components. As such it indicates that a high-intensity statin alone could halt necrotic (or lipid) core progression. Future studies might focus on the question whether regression is possible in lesions with a higher baseline atheroma burden. |
Guest Editor
This paper was guest edited by William Wijns, MD, PhD; Cardiovascular Research Centre Aalst, OLV Clinic, Aalst, Belgium, The Lambe Institute for Translational Medicine and Curam, National University of Ireland, Galway, Ireland and Saolta University Healthcare Group, Galway, Ireland.
Funding
IBIS-3 was sponsored by AstraZeneca (Wilmington, DE, USA), by Infraredx (Burlington, MA, USA) and by Volcano Corporation (San Diego, CA, USA) through unrestricted research grants to the ErasmusMC, Rotterdam, The Netherlands.
Conflict of interest statement
The study was initiated, designed, conducted, interpreted, and reported by the authors under the leadership of the principal investigator Prof. Dr. P.W. Serruys, independently of these sponsors. The above-mentioned funding was directly granted to the institution of the ErasmusMC, Rotterdam, The Netherlands. Cardialysis BV, Rotterdam, The Netherlands, was only contracted by the ErasmusMC for its independent core laboratory services. The authors, employees of the ErasmusMC and in case of Dr. Garcia-Garcia only of Cardialysis, declare no conflicts of interest. The Guest Editor has no conflicts of interest to declare in relation to this specific paper.
Supplementary data
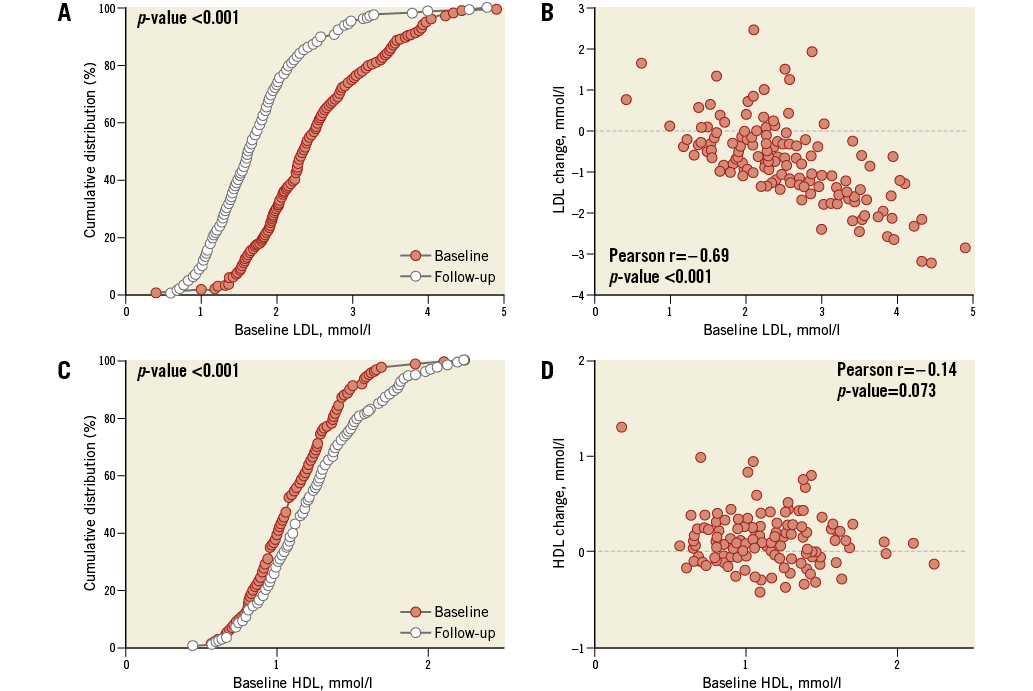
Online Figure 1. Serum cholesterol levels at baseline and follow-up.High-intensity rosuvastatin therapy during a median follow-up of 372 days resulted in a significant decrease in serum LDL-c (A) and increase in HDL-c levels (C), despite the fact that 95% of the patients were already on standard-of-care statin therapy at baseline. The degree of reduction in LDL-c was related to the baseline LDL-c level (B). Such a correlation was not observed with respect to HDL-c (D). HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol
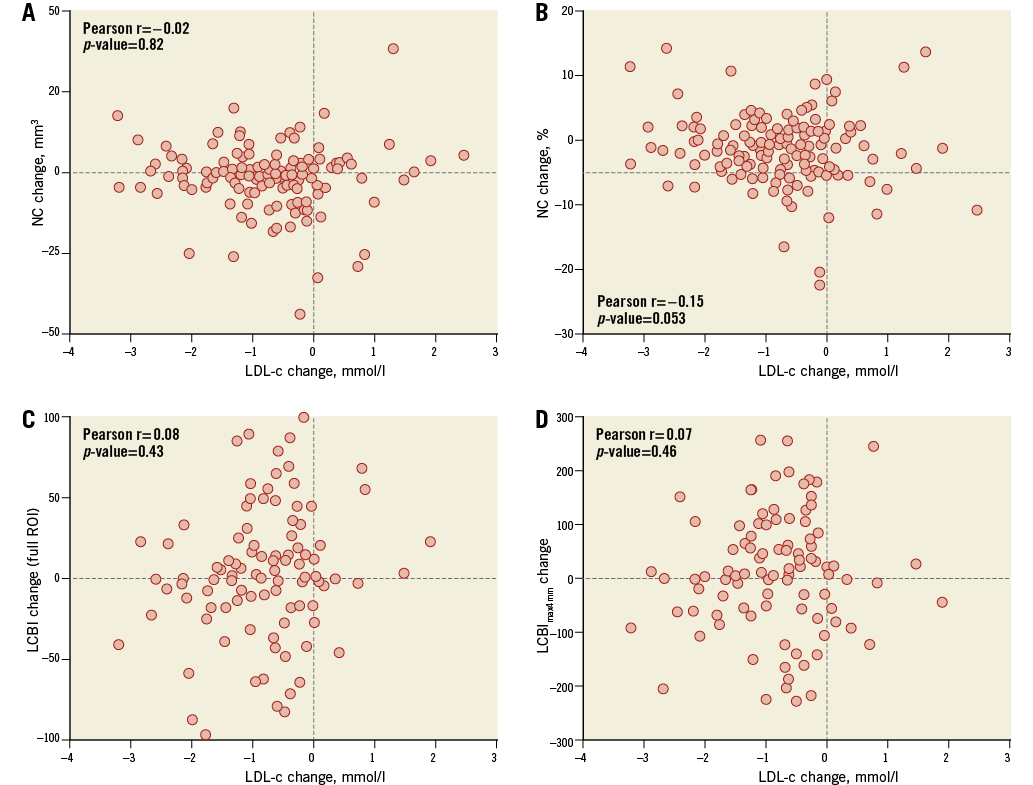
Online Figure 2. Change in LDL-c in relation to change in necrotic core and LCBI. Changes in NC and LCBI were independent of changes in LDL levels under rosuvastatin therapy. A) NC volume; B) NC percentage; C) Full region of interest; D) LCBImax4mm LCBI: lipid core burden index; LCBImax4mm: the 4 mm segment with the highest LCBI; LDL-c: low-density lipoprotein cholesterol; NC: necrotic core

