Abstract
Aims: Acute coronary syndromes (ACS) are often caused by rupture of non-flow limiting “vulnerable” atherosclerotic plaque, characterised by a large necrotic core pool and a thin, inflamed fibrous cap that are unidentifiable with diagnostic coronary angiography. The implementation of novel invasive imaging modalities, such as intravascular ultrasound-virtual histology (IVUS-VH) and near-infrared spectroscopy (NIRS), could help identify high-risk patients who are in need of aggressive medical therapy. The intake of high dose rosuvastatin has been shown to reduce plaque containing necrotic core in the carotid arteries, however it remains unknown whether there is a similar effect in the coronary arteries.
Methods and results: The IBIS-3 study is a single centre, non-randomised study designed to evaluate the ability of 12-months high dose rosuvastatin treatment (40 mg daily po) to reduce the necrotic core of a non-intervened coronary segment assessed with IVUS-VH. All patients undergoing diagnostic angiography or percutaneous coronary intervention (PCI) are eligible for participation. Recruitment started in early 2010 and will continue until 350 patients are included. The effect on the lipid core containing plaque will be assessed with near-infrared spectroscopy (NIRS) at 12-months. In addition, multiple biomarkers will be measured and their levels correlated with the imaging results.
Conclusions: IBIS-3 will assess the efficacy of high dose rosuvastatin to reduce the necrotic core in a coronary segment of patients who have undergone diagnostic angiography or PCI.
Abbreviations
ACS: acute coronary syndrome
CPK: creatine phosphokinase
HDL: high-density lipoprotein
hs-CRP: high sensitivity C-reactive protein
IVUS-VH: intravascular ultrasound-virtual histology
LDL: low-density lipoprotein
LCP: lipid core containing plaque
NIRS: near-infrared spectroscopy
PCI: percutaneous coronary intervention
ROI: region of interest
Introduction
Cardiovascular disease remains the leading cause of death in the Western world. Over time, it has become clear that coronary atherosclerosis is a complex multifactorial process caused by lipid accumulation in combination with several immunological and inflammatory reactions1-3. Coronary angiography can detect and evaluate the extent of coronary artery disease, however being a luminogram technique it does not provide a comprehensive assessment of true atherosclerotic burden.
It is atherosclerotic lesions with a thin inflamed fibrous cap overlying a lipid-rich necrotic core, and not the degree of angiographic stenosis per se, that have been shown to be related to an increased incidence of cardiac events4. Approximately 60% of all acute coronary syndromes (ACS) are thought to be caused by the rupture of such vulnerable plaques5. Therapeutic interventions that can lead to regression of or stabilise vulnerable plaque and consequently reduce the incidence of ACS need to be assessed in vivo. The visualisation of these dynamic processes with intravascular ultrasound-virtual histology (IVUS-VH), based on pattern classification of backscattering ultrasound signal, proved to be highly accurate in assessing four different plaque compositions i.e., fibro-fatty, fibrous tissue, dense calcium and necrotic core6,7.
The STELLAR study showed that rosuvastatin decreased low-density lipoprotein (LDL) cholesterol and increased high-density lipoprotein (HDL) cholesterol with a higher potency when compared to other statins8. In addition, the decrease of LDL cholesterol levels resulted in a significant regression of coronary atherosclerosis and also reduced the lipid-rich necrotic core in the carotid arteries9,10. Even healthy people with non-elevated LDL cholesterol levels and a raised inflammatory marker (hs-CRP) had less major adverse cardiac events (MACE) with rosuvastatin intake compared to placebo11.
The IBIS-2 study showed that 12 months of treatment with 160 mg oral darapladib (an oral LP-PLA2 inhibitor) halted the progression of necrotic core volume in the coronary arteries compared to an increase in the placebo group, resulting in a significant treatment benefit12. Moreover, the necrotic core expanded in the placebo group despite a high-level standard of care treatment.
In summary, rosuvastatin is highly effective in reducing LDL cholesterol levels and increasing HDL cholesterol levels, which is reflected in the regression of lipid-rich necrotic core in the carotid arteries. However, the effect of rosuvastatin use on the necrotic core in the coronary arteries remains to be evaluated. The IBIS-3 study has been designed to address this question.
Methods
Study design and objective
The IBIS-3 study is a prospective, investigator-initiated, single centre, non-randomised study. The study has been designed to evaluate the ability of a high-dose rosuvastatin (40 mg daily po) to reduce the necrotic core of a non-intervened coronary segment (study vessel) assessed with IVUS-VH at 12-months. Particularly, the change in necrotic core volume within the region of interest (ROI) from baseline to one-year will be assessed by offline IVUS-VH7. In addition, the change in the near-infrared spectroscopy (NIRS) derived lipid core burden for the entire ROI will be assessed13,14. The potential relationship between biomarkers associated with plaque progression (such as markers of vascular inflammation, endothelial activation and thrombosis) will be explored as well12,15,16.
Study population
A total of 350 IBIS-3 patients, referred to our institution for elective or urgent diagnostic angiography or percutaneous coronary intervention (PCI), in whom a non culprit, non-treated vessel was judged analysable will be prospectively enrolled. All patients included will be 18 years or older, with stable/unstable angina pectoris, documented silent ischaemia or an acute myocardial infarction (STEMI or non-STEMI). Coronary segments that are bypassed/stented, perfuse a scarred myocardial region or have a minimal lumen diameter <2 mm or a diameter stenosis >50% by angiographic visual estimation in the segments to be analysed will be excluded (Figure 1).
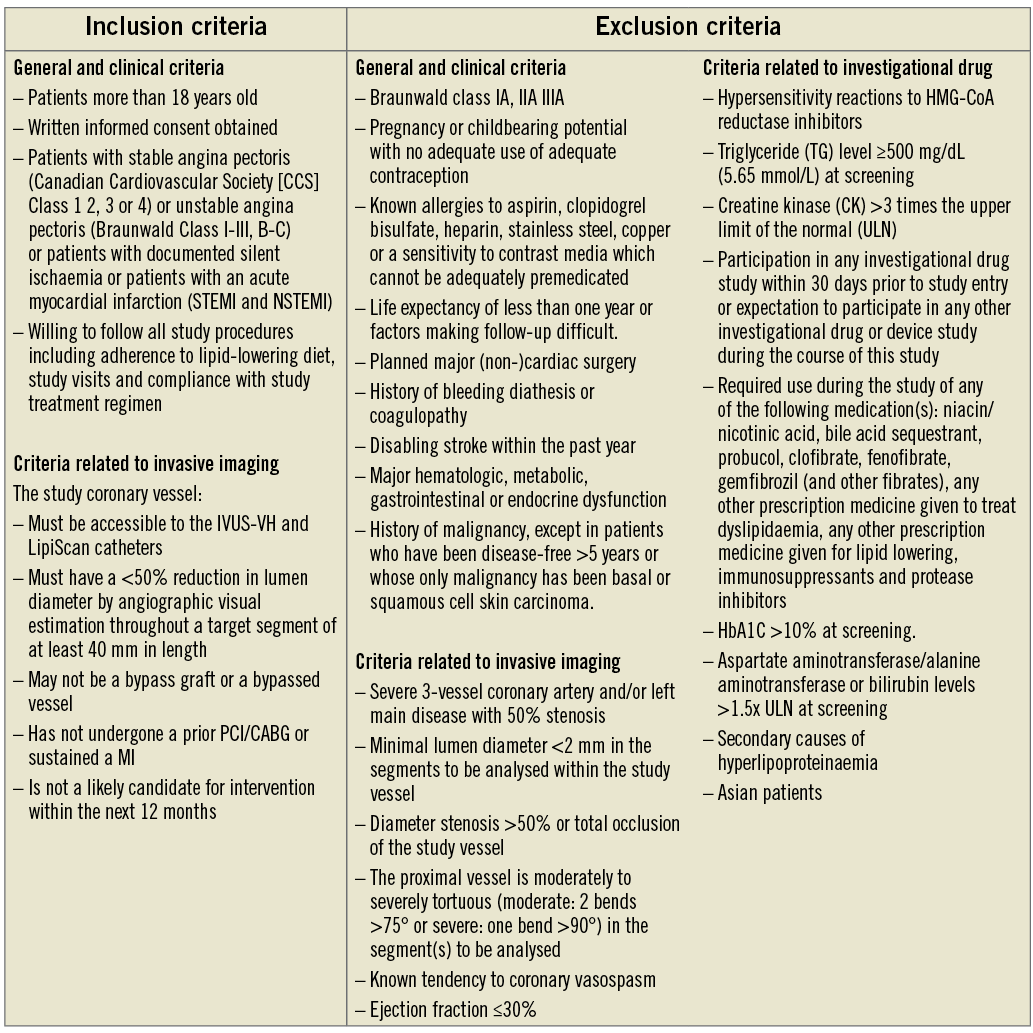
Figure 1. Inclusion and exclusion criteria of the IBIS-3 study.
The patients will receive the first dose of rosuvastatin 10 mg po within two weeks of enrolment. The dose of rosuvastatin will be titrated up to 40 mg within 30 days (Figure 2). The study is conducted under the guidance of the Institutional Review Board and written informed consent is obtained from all patients.
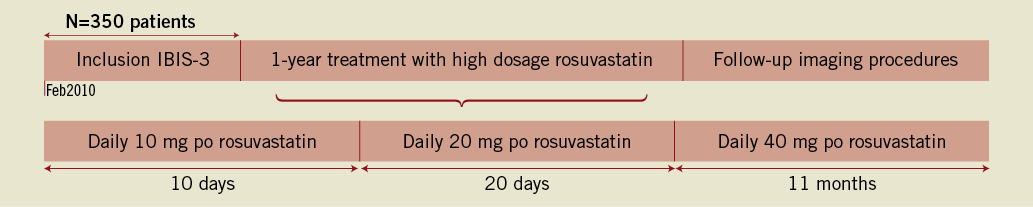
Figure 2. Timetable of the IBIS-3 study.
Study procedures
After arterial sheath insertion, blood samples of 50 ml will be collected at baseline and after 12-months treatment with rosuvastatin. All imaging procedures are performed immediately after the coronary angiography/PCI procedure. The study coronary vessel must have a <50% reduction in lumen diameter by angiographic visual estimation throughout a target segment of at least 40 mm in length. The ROI is defined as the longest coronary segment between two clearly identifiable landmarks (i.e., side branches). The length of the ROI is determined by the analysable matched segments on IVUS-VH and NIRS.
IVUS-VH and NIRS acquisition
IVUS-VH has been shown to have a 80-93% in vitro accuracy when used to identify fibrous, fibro-fatty, dense calcium, and necrotic core-containing plaque types6. Briefly, IVUS-VH makes use of autoregressive spectral analysis of IVUS radiofrequency backscattering data to construct “tissue maps” that are associated with a particular spectrum of the radiofrequency signal and assigned colour codes4. The coronary vessel will be imaged with a 20 MHz IVUS catheter (Eagle-Eye®; Volcano Therapeutics, Rancho Cordova, CA, USA) at a continuous motorised pullback speed of 0.5 mm/sec (R-100 pullback device; Volcano Therapeutics). The acquired images will be stored in a digital database on a dedicated console (In-Vision Gold; Volcano Therapeutics) (Figure 3).
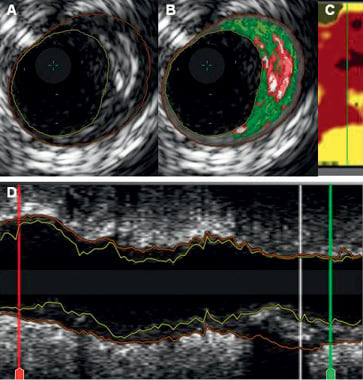
Figure 3. Images from an IBIS-3 patient including intravascular ultrasound (IVUS) greyscale (panels A and D), IVUS virtual histology (VH) (panel B) and LipiScan (panel C) on the same coronary segment. On the L-view (panel D), the region of interest is demarcated by the red and green lines (between two anatomical landmarks). The white line in panel D indicates the location of the frames shown in panels A and B. Panel A shows an eccentric and mixed plaque. The corresponding VH frames show a necrotic core (red) rich plaque with a large confluent necrotic core area abutting the lumen at 3 o’clock. By LipiScan (panel C) this plaque (green line) was characterised as a lipid-rich (cholesterol-containing) plaque (yellow colour)
NIRS is a novel catheter-based imaging modality that was validated in a large prospective autopsy study for the detection of lipid core containing plaque (LCP) with good agreement with histology13. The acquisition of imaging data will be performed by a motorised catheter pullback at a speed of 0.5 mm/sec, starting distal to a side branch. The amount of LCP for each scanned arterial segment in the ROI is displayed as a chemogram, with the x-axis indicating the pullback position in millimetres and the y-axis the circumferential position of the measurement in degrees (comparable to a coronary vessel split open along the longitudinal axis), while the probability of LCP present will be coded on a binned colour scale from red to yellow (0 for red and 1 for yellow), in which pixels that exceed a pre-specified threshold of 60% turn to yellow, suggesting the presence of a LCP. The pixel will appear black if it lacks enough data. Our previous study showed that plaques coded as yellow were associated with significantly larger plaque sizes compared to those coded as red and that it was weakly correlated with necrotic core17. The NIRS-derived lipid core burden index (LCBI) score is calculated by multiplying the fraction of valid yellow pixels by 1,000 and summarises the amount of LCP in the imaged coronary section on a 0 to 1,000 scale (Figure 4).
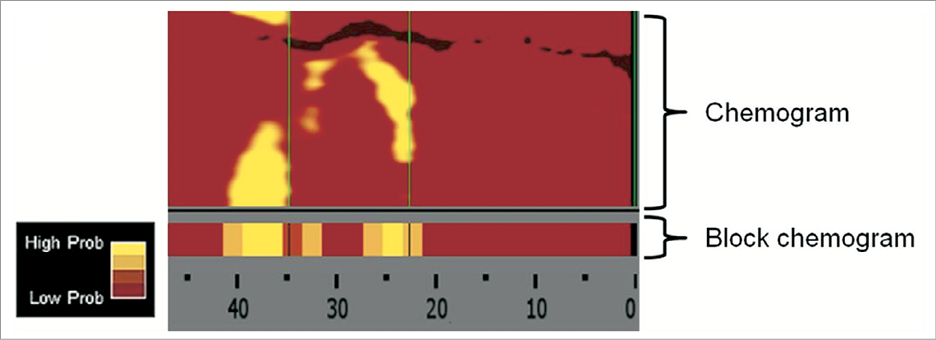
Figure 4. The chemogram is a map of the scanned arterial segment, with the x-axis indicating the pullback position in millimeters and the y-axis the circumferential position of the measurement in degrees. The block chemogram provides a summary of the data in 2 mm segments. The algorithm displays the probability of lipid core containing plaque (LCP) at the interrogation site by using a false colour scale from red (low probability) to yellow (high probability) The lipid core burden index (LCBI) provides a semi-quantitative metric summary of the total LCP detected and can be calculated for the full chemogram or regions of interest.
Follow-up
All enrolled patients will be followed up until the study is completed. The study patients will be contacted by phone at three weeks and 11 months to assess clinical status and any side effects. A clinical visit is planned at two months and 12 months after study enrolment. Invasive imaging will be repeated at 12 months. The imaging data of patients who needed urgent cardiac catheterisation <12 months but >6 months after randomisation will be included in the analysis. If the patient cannot be interviewed at the outpatient clinic at 12 months, information concerning possible events is obtained by phone contact with the patient, family or his general practitioner. The patient is considered lost to follow-up if this communication is unsuccessful.
Endpoints
The primary endpoint for this study is the change in necrotic core volume (mm3) of a non-intervened coronary segment after 12-months treatment with 40 mg rosuvastatin, as assessed by IVUS-VH. The secondary endpoints are: 1) the change in percentage necrotic core of the total plaque in the ROI; 2) the change in total atheroma volume within the ROI; 3) the change in necrotic core (mm3) within the worst 10 mm coronary segment; 4) the change in percentage necrotic core of the total atheroma in the worst 10 mm coronary segment; 5) the change in total atheroma volume (mm3) within the worst 10 mm coronary segment; 6) the change in the number of cholesterol-containing block chemograms in the ROI; and 7) the change in the NIRS-derived “lipid core burden index” for the entire ROI. Laboratory analyses, representing more classic markers of inflammation and vulnerability will be performed to explore the association between biomarkers and plaque, and necrotic core progression.
Adverse events and additional safety assessments
The incidence of side effects is expected to be dose-dependent. Therefore, liver function tests and creatine phosphokinase (CPK) will be measured within two months after starting the treatment in combination with an electrocardiogram. The dose of rosuvastatin will be lowered or discontinued in the case of a serum transaminase concentration more than three times or a CPK level of more than five times the upper limit of the reference value.
All study patients will be monitored for (serious) adverse events from enrolment to 52 weeks after enrolment. Patients may be discontinued from study treatment and assessments at any time at the discretion of the investigator(s), if it is felt that this action is in the best interests of the patient.
Statistical analysis
Data analysis will be performed on an intent-to-treat basis of all subjects who receive study medication. All patients with analysable baseline and follow-up IVUS-VH images will be evaluated. Statistical inference will be based on the primary endpoint being change in necrotic core volume from baseline to follow-up. The assumptions for the sample size calculation are based on data from the IBIS-2 study: 1) two-sided type 1 error rate of 0.05; 2) attrition rate of 15%; 3) difference of 2.5 mm3 of necrotic core volume with a standard deviation of 13.9 mm3 will lead to a power of 90%12. A total of 350 patients need to be included to obtain at least 300 imaging evaluable patients at baseline and follow-up. The treatment effect will be tested with the paired-t test, based on a two-sided significance level of 0.05. Kaplan-Meier curves will estimate the cumulative adverse cardiac events. All statistical analysis will be performed with SPSS for Windows version 17 (SPSS Inc., Chicago, IL, USA).
Discussion
The IBIS-3 study has been designed to test the hypothesis that a high dose of rosuvastatin significantly decreases or halts the progress of the necrotic core volume present in a non-intervened coronary segment as assessed by IVUS-VH. The relevance of this hypothesis is related to the fact that atherosclerotic plaque with a thin inflamed fibrous cap and a large necrotic core pool is accountable for nearly 60% of all ACS5.
The current medical treatment strategy for patients undergoing PCI includes the use of aspirin, clopidogrel and a statin17. Over the last few decades, evidence from randomised trials has shown that 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, so-called “statins”, reduce LDL cholesterol levels and thereby also lower the occurrence of major vascular events by 21% for every 1 mmol/l (39 mg/dl) drop in LDL cholesterol18. Rosuvastatin has a relatively higher binding capacity to HMG-CoA reductase inhibitors compared to other statins and therefore resulted in a more pronounced LDL cholesterol reduction of patients with hypercholesterolaemia in the STELLAR study8,19. In the JUPITER study, the use of rosuvastatin significantly reduced the occurrence of major adverse cardiac events in patients with normal LDL cholesterol levels and raised high sensitivity C-reactive protein as compared to placebo treatment11. At the same time, the safety and tolerability of rosuvastatin 5-40 mg po is still comparable to simvastatin 10-80 mg po20. Although the occurrence of adverse events are low, primary reasons for statin discontinuation are statin-induced liver problems and myalgia.
On the basis of the above data, it seems justified to study the effects of rosuvastatin on the existing atherosclerotic plaque volume and composition. In order to assess (intra)vascular changes in the carotid and coronary arteries after rosuvastatin, a variety of (non)invasive imaging modalities have been used in different randomised trials. The METEOR trial, which studied 984 patients, showed that patients randomised to treatment with rosuvastatin had a decline of 0.0014 mm per year of the carotid intima-media thickness while the placebo-group had an increase of 0.0131 mm per year (p<0.001), as assessed with ultrasound over two years21. Positive results were also found in the ORION trial, which showed a reduction in the lipid-rich necrotic core of carotid atherosclerotic plaque as assessed with magnetic resonance imaging10. Additionally, invasive imaging of the coronary arteries with IVUS at baseline and two-years, showed that a high dose of rosuvastatin had beneficial effects on all pre-specified endpoints9. We hypothesise that a significant effect on the necrotic core volume will be shown with one-year treatment with high dose rosuvastatin, which is comparable with the effect of darapladib in the IBIS-2 study. If proved, it would be interesting to further study the synergistic action of both medications on the necrotic core.
The first potential limitation of the IBIS-3 study is the fact that it is not a randomised trial. Secondly, there are several exclusion criteria that limit the widespread use of a high dose of rosuvastatin. Finally, significant differences in necrotic core volume are expected mainly in patients with an ACS who are statin naïve. Based on our prior experience in performing “real-world” registries, we expect that only a minority of the included patients will be statin naïve; the additional or incremental effect of high dose rosuvastatin might be less evident in patients already treated with the standard care22,23.
In conclusion, the IBIS-3 study will be the first study to evaluate prospectively the effect of a high dose of rosuvastatin on the necrotic core existing in atherosclerotic plaque in non-intervened coronary arteries. If the hypothesis of the IBIS-3 study is confirmed, it could lead to a different understanding of treatment modulation of plaque behaviour in patients with coronary artery disease. The first results are to be expected mid 2013.
Acknowledgements
C. Simsek was supported by a research grant from the “Nederlandse Hartstichting”, The Hague, The Netherlands (2009B091).
Funding sources
This investigator-driven study is supported by AstraZeneca. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents. This study has been registered in “The Netherlands trial register; NTR2872”.
Conflict of interest statement
This study is supported by AstraZeneca, Wilmington, DE, USA. J. Raichlen is an employee of and owns stock in AstraZeneca. All other authors have no conflict of interest to declare.

