Abstract
Aims: The aim of this study was to evaluate the effect of low-dose versus high-dose rosuvastatin therapy on the necrotic core (NC) content in coronary plaques of angiographic non-significant lesions as assessed by intravascular ultrasound - virtual histology.
Methods and results: Eighty-seven statin-naive patients with ST-segment elevation myocardial infarction (STEMI) were randomised to 5 mg or 40 mg rosuvastatin. The volume of each plaque component (necrotic core, fibrous tissue, fibro-fatty, and dense calcium) was assessed at baseline and after 12 months of follow-up. Baseline low-density lipoprotein (LDL) cholesterol level was reduced by 31.8% in the low-dose group (from 3.1±0.7 mmol/l to 2.0±0.4 mmol/l, p<0.001) vs. 49.0% in the high-dose group (from 3.1±1.0 mmol/l to 1.6±0.7 mmol/l, p<0.001) (p=0.001 between groups). The plaque component necrotic core was reduced by 7.6% in the low-dose group (44.6±38.2 mm3 vs. 41.2±40.3 mm3, p=0.29) compared to 14.2% in the high-dose group (47.4±38.2 mm3 vs. 40.7±34.4 mm3, p=0.003) (p=0.38 between groups).
Conclusions: In statin-naive patients with STEMI, rosuvastatin therapy for 12 months resulted in significant reduction in LDL-cholesterol; however, a significant decrease in necrotic core volume was only seen in the high-dose group. (Clinicaltrials.gov Identifier: NCT01058915).
Introduction
Various studies have shown that rupture of an unstable vulnerable plaque and thrombus formation is one of the most important causes of acute coronary syndrome (ACS)1,2. A typical lesion has a large necrotic core with a thin fibrous rupture-prone cap covering the lesion3. Despite only one single lesion being clinically active at the time of ACS, the syndrome nevertheless seems associated with overall coronary instability4. To describe the extent and severity and to evaluate regression and progression of coronary atherosclerotic plaques, greyscale intravascular ultrasound (IVUS) has been an invaluable tool. The technique is highly reproducible5,6 and has been used in the evaluation of different plaque-modifying interventions7,8 although it only allows the determination of geometrical data9. IVUS-virtual histology (IVUS-VH) uses spectral analysis of radio-frequency data obtained by greyscale IVUS which gives the opportunity to achieve plaque compositional data besides the geometrical data provided by greyscale IVUS. The technique distinguishes four different tissue types: fibrotic, fibro-fatty, necrotic core and dense calcium which are colour-coded with green, greenish-yellow, red and white, respectively, and thereby the technique is able to identify subclinical and clinical high-risk plaques10,11. The technique is validated by in vitro and in vivo studies12,13 and found highly reproducible14. Patients with coronary artery disease benefit from lipid-lowering treatment with HMG-CoA reductase inhibitors (statins)15; however, the influence of statin therapy on changes in coronary plaque components has not previously been studied in high-risk statin-naive patients presenting with a ST-segment elevation myocardial infarction (STEMI).
The aim of this study was to examine the effect of low-dose versus high-dose rosuvastatin therapy in a twelve-month treatment period on the plaque composition in a non-infarct-related artery in statin-naive patients with STEMI.
Methods
STUDY POPULATION
The Influence of Intensive Lipid Lowering Treatment Compared to Low Lipid Lowering Treatment on Plaque Composition Assessed by Virtual Histology in Patients with ST-Segment Elevation Acute Myocardial Infarction (VIRHISTAMI) trial was a single-centre, single-blinded randomised study. From November 2007 to June 2009, 87 statin-naive patients with STEMI treated with primary percutaneous coronary intervention (PCI) were enrolled in this study. The inclusion criteria were: 1) STEMI; 2) no prior treatment with statins or other lipid lowering drugs; and 3) a non-significant lesion in one of the two non-culprit coronary arteries (angiographic diameter stenosis >20% and <50%). The exclusion criteria were: 1) age below 18 or above 81 years; 2) unconscious patients; 3) serum creatinine >176 μmol/L; 4) hypothyroidism (TSH >1.5 x ULN [upper limit of normal]); 5) current liver disease (ALAT >2 x ULN); 6) unexplained creatine kinase >3 x ULN; 7) alcohol or drug abuse within the last five years; 8) prior myopathy or serious hypersensitivity reaction caused by statins; 9) women with child-bearing potential who were not using chemical or mechanical contraception; 10) pregnant or breastfeeding women; 11) history of malignancy unless a disease-free period of more than five years was present; 12) participation in another randomised trial; 13) treatment with cyclosporine or fibrates. All patients provided written, informed consent, and the protocol was approved by the local institutional review board (The Scientific Ethics Committee for the County of Fuenen, Denmark, case no. VF-20060060). The study was performed in accordance with the rules for good clinical practice (GCP) and monitored by the GCP-department of Odense University Hospital and the Danish Medicines Agency.
At baseline, eligible patients were randomised to treatment with either 5 mg rosuvastatin or 40 mg rosuvastatin (1:1 ratio) for 12 months. The physicians in the study group were blinded with regard to the laboratory values and the randomised statin group during the study. The cholesterol levels were monitored by a study nurse not otherwise involved in the study to achieve the optimal treatment level of total-cholesterol <4.5 mmol/l and LDL-cholesterol <2.5 mmol/l and, if not, the patients in the low-dose group had their dose increased with 5 mg rosuvastatin every third month until the goal was achieved. If the patients in the high-dose group did not reach the treatment goal on 40 mg rosuvastatin they were excluded from the study. Blood pressure was obtained after 10 minutes rest with the patient in supine position. (Clinicaltrials.gov Identifier: NCT01058915).
ENDPOINTS
The primary endpoint was the change in necrotic core volume in a not previously revascularised or infarct-related coronary artery with an angiographic insignificant lesion. The secondary endpoints were changes in volumes of: fibrotic tissue, fibro-fatty tissue and dense calcium in the entire segment of interest, the changes in plaque components in the most diseased 10 mm segment, the changes in percentage atheroma volume (PAV) and total atheroma volume (TAV) in the entire segment of interest.
IVUS-VH ACQUISITION
The following working day after the primary PCI the patients were readmitted to the catheterisation laboratory for an angiogram and IVUS-VH was performed of a non-infarct-related coronary artery. IVUS images were acquired after intracoronary administration of 200 µg nitroglycerine and heparin 5,000 IU using an Eagle Eye Gold 2.9 Fr 20 MHz IVUS catheter and a Volcano s5 console (Volcano Corp., Rancho Cordova, CA, USA). The catheter was placed 20 mm distal to an angiographic insignificant lesion and a motorised pullback was performed with a speed of 0.5 mm/s until the ostium of the coronary artery. The VH image acquisition was ECG-gated triggered by the R-wave peaks. The patients were scheduled to undergo a new IVUS-VH examination after 12 months with examination of the same segment of interest.
IVUS-VH ANALYSIS
The IVUS-VH data were stored on DVDs for offline analyses. The segments for analysis were identified by use of an anatomic landmark and the baseline and 12-month follow-up examinations were matched before analysis. The most diseased 10 mm segment at baseline within the total segment was identified and matched as well. The analyses were performed by a dedicated operator (RE) who was blinded both to the treatment groups and to the baseline and 12-month examinations. The reproducibility of the virtual histology analysis has been tested previously and can be done successfully14. The analyses were performed with VIAS-software (Volcano Corp., Rancho Cordova, CA, USA). The technique uses spectral analysis of radio-frequency data obtained by greyscale IVUS to classify plaque components according to their content of fibrous tissue, fibro-fatty tissue, necrotic core and calcium. The VH images were acquired on top of the R-wave peaks and every frame was analysed. The greyscale IVUS analyses were performed with EchoPlaque (INDEC Systems, Inc., Mountain View, CA, USA). For every 1 mm of the segment lumen cross-sectional area (CSA) and the external elastic membrane (EEM) CSA was measured. The PAV was calculated as: (∑[EEM CSA–lumen CSA]/∑EEM CSA)*100 and TAV was calculated as: ∑(EEMCSA–lumenCSA).
STATISTICAL ANALYSIS
The statistical analyses were performed with Stata 11.0 (StataCorp, College Station, TX, USA). Categorical data are presented as frequencies and percentages and were compared using chi-square test. Continuous data are presented as mean±SD and were compared using a Student’s t-test or Mann-Whitney rank sum test when normality testing failed. The within-subject changes from baseline were evaluated using a paired t-test. The unpaired t-test was used for comparisons between groups. A two-sided p-value of <0.05 was considered statistically significant. The sample size calculation was based upon an estimation approach because the effects of statins on plaque components were unknown. We estimated a change in necrotic core of 25% in the high-dose group and 15% in the low-dose group with a SD of 16.5%, alpha error of 0.05, and a power of 80%. Thus, a minimum of 88 patients was pre-specified for enrolment. IVUS-VH data will only be available for patients returning for a follow-up IVUS after 12 months.
Results
PATIENT POPULATION
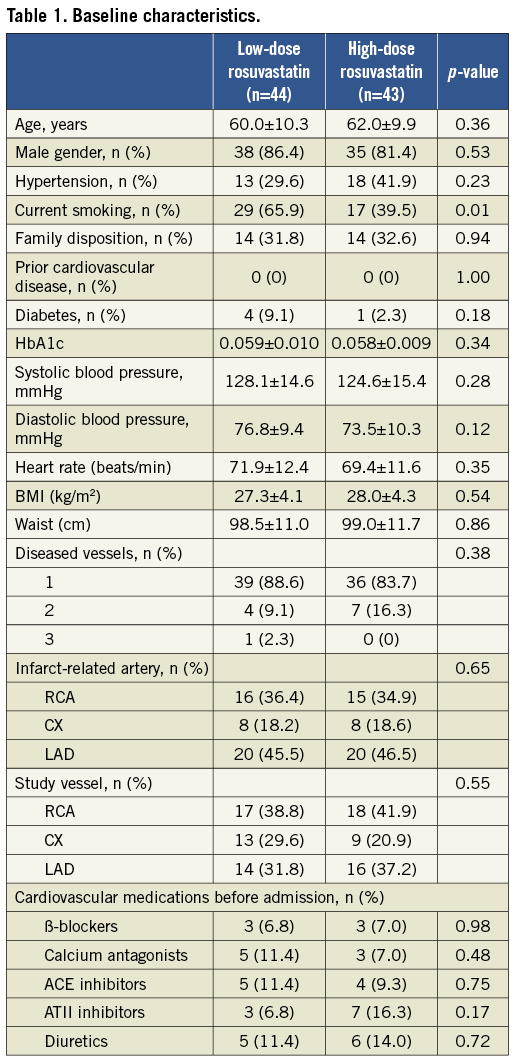
In total, 87 patients were randomised to rosuvastatin 5 mg (low-dose, n=44) and rosuvastatin 40 mg (high-dose, n=43). The study was completed by 82 patients but only 77 patients had both baseline and follow-up examinations for analysis (Figure 1). Prior to the index STEMI none of the patients had suffered an acute myocardial infarction. The time to follow-up was comparable: 374.2±12.4 days vs. 369.3±31.1 days (p=0.37) in the two groups. The baseline characteristics were similar in both treatment groups except for patients in the low-dose rosuvastatin group who were more likely to be current smokers. Patient characteristics are presented in Table 1. The study-drug was well tolerated and in the twelve-month treatment period no adverse events were found in any of the groups.
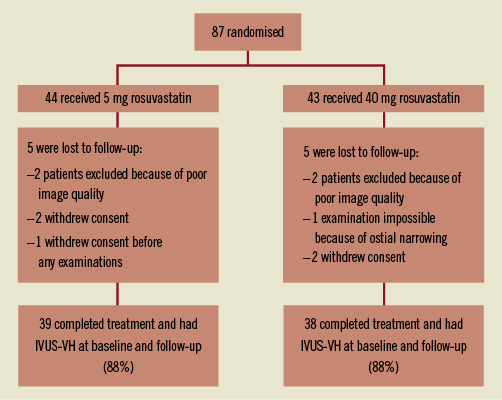
Figure 1. Flow chart of patients included in the study.
LIPIDS
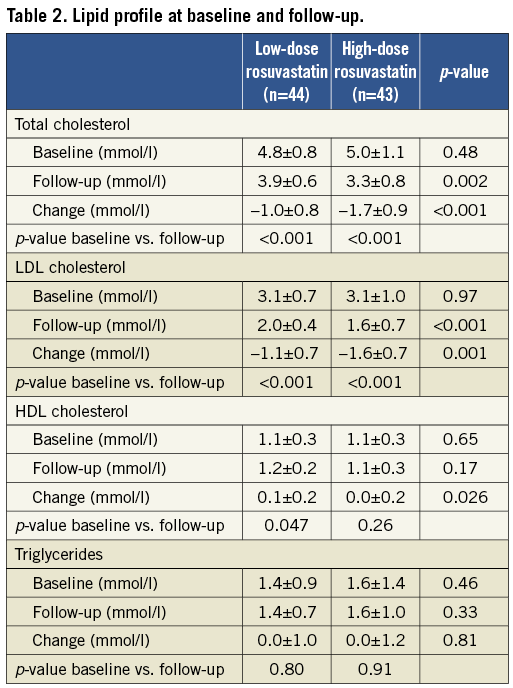
The desired levels of total-cholesterol <4.5 mmol/l and LDL-cholesterol <2.5 mmol/l were not reached in 14 and three patients in the low-dose group (5 mg rosuvastatin) at three and six months, respectively. Therefore, the rosuvastatin dose was increased to a mean value of 8.2±6.2 mg at follow-up in the low-dose group. In the high-dose group (40 mg rosuvastatin), all patients reached the desired cholesterol levels. Total-cholesterol decreased with 18.6% vs. 31.9% (p<0.001 between groups) and LDL-cholesterol decreased with 31.8% vs. 49.0% (p<0.001 between groups) in the low-dose group versus the high-dose group, respectively. The HDL-cholesterol increased in the low-dose group but decreased in the high-dose treatment group: 12.5% vs. –1.7% (p=0.029 between groups), and the triglycerides changed with 2.7% vs. –1.3% (p=0.49 between groups), respectively (Table 2).
IVUS-VH FINDINGS
One segment per patient with a mean length of 32.7±18.1 mm was analysed. The plaque components at baseline did not differ significantly between the two groups (Table 3). Overall, the necrotic core volume was reduced by 11.0% (p=0.011) and the fibrous tissue volume was reduced by 4.4% (p=0.07), whereas the fibro-fatty tissue and dense calcium volume were unchanged. In the low-dose group, the fibrous tissue volume was reduced significantly by 8.1% (p=0.01), and necrotic core volume was reduced by 7.6% (p=0.29), whereas the volumes of fibro-fatty tissue and dense calcium did not change significantly. In the high-dose group the necrotic core volume decreased significantly by 14.2% (p=0.003) and fibro-fatty tissue volume increased significantly by 21.8% (p=0.03), whereas the volumes of fibrous tissue and dense calcium did not change significantly (Figure 2A). When looking at the most diseased 10 mm segment, the results were confirmed except for the increase in fibro-fatty tissue volume in the high-dose group that did not reach statistical significance (Table 4, Figure 2B). Baseline HDL or changes in HDL during treatment were not related to changes in plaque components.
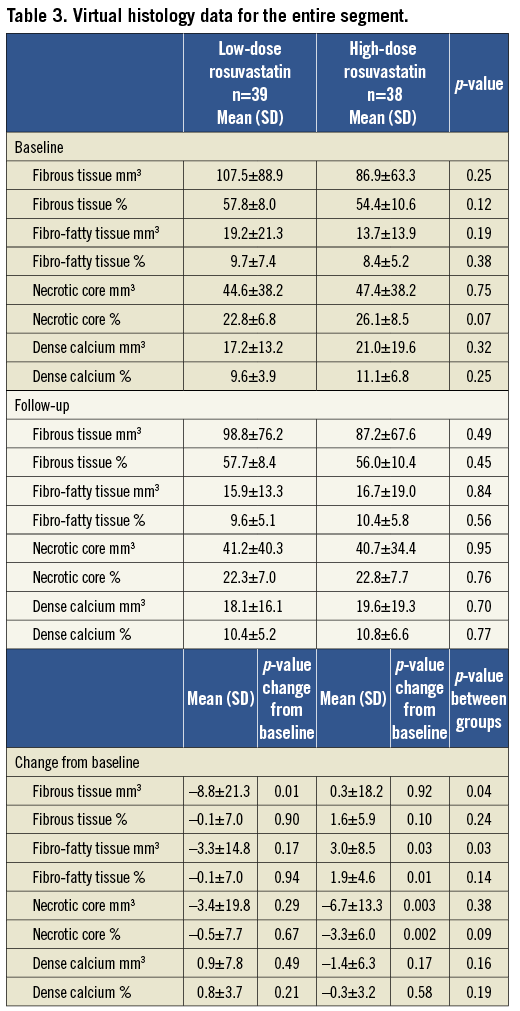
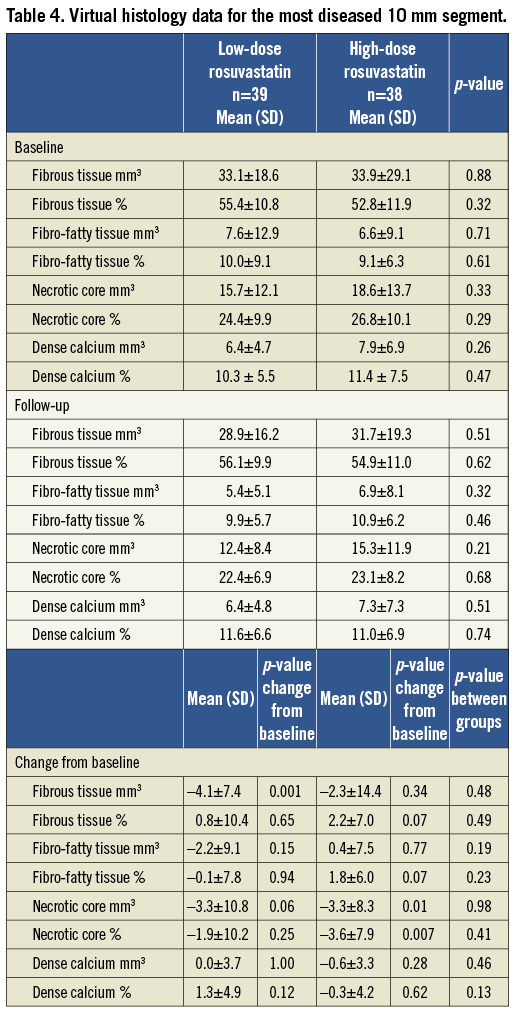
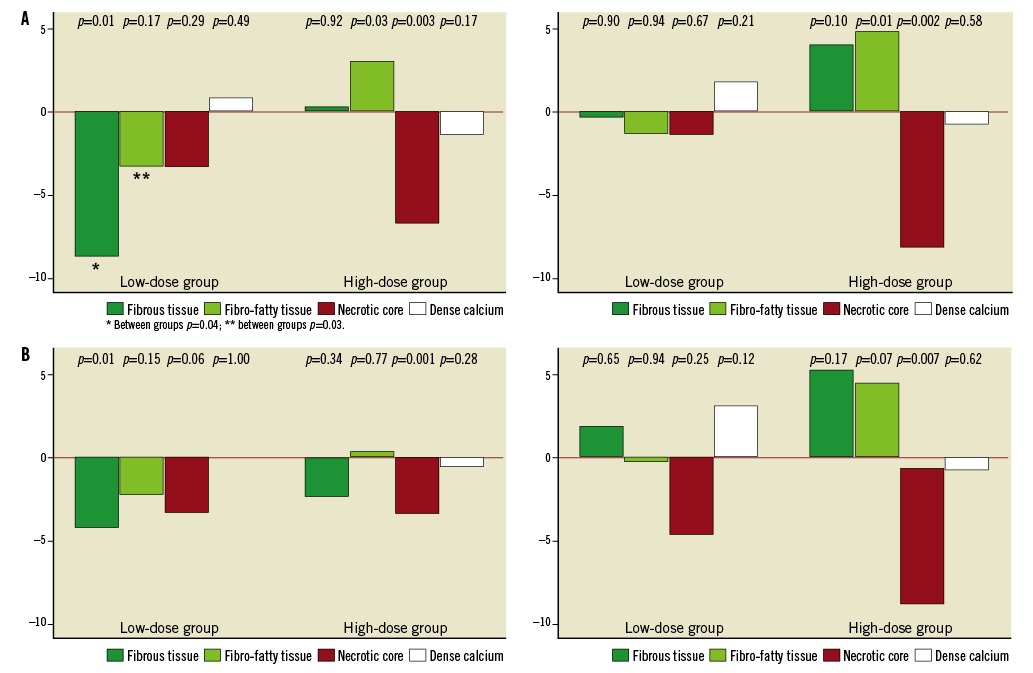
Figure 2. A) Change in the absolute and relative plaque composition in the entire segment of interest. B) Change in the absolute and relative plaque composition in the most diseased 10 mm segment.
In the entire segment of interest the TAV decreased from baseline to follow-up [–18.7±27.8 mm3 (–3.3%)] (p<0.001) vs. [–12.2±30.1 mm3 (-2.8%)] (p=0.017), between groups p=0.33 in the low-dose group and the high-dose group, respectively. The PAV decreased (–1.3±2.9%) (p=0.007) vs. (–0.3±4.2%) (p=0.63), between groups p=0.24.
Discussion
In the present serial IVUS-VH study, treatment with rosuvastatin for twelve months resulted in plaque regression by a reduction in necrotic core volume in statin-naive patients. Patients treated with a high-dose rosuvastatin (40 mg) seemed to benefit more with a higher reduction in necrotic core volume without a concomitant higher reduction in total atheroma volume. The plaque components fibro-fatty tissue, fibrous tissue and dense calcium did not change significantly overall, but in patients treated with high-dose rosuvastatin, where the highest reduction in necrotic core volume was seen, the volume of fibro-fatty tissue increased significantly.
The effects of statins on IVUS-VH plaque composition have recently been studied for rosuvastatin, simvastatin, fluvastatin, pitavastatin and atorvastatin16-18 and for a direct lipoprotein-associated phospholipase A2 inhibitor19. As in the present study, the three other statin studies demonstrated a significant regression of plaque volumes on greyscale IVUS16-18, which is comparable to the effects of statins on plaque volumes in greyscale IVUS regression/progression studies7,8,20.
The percentage of necrotic core in our study is similar to the results of the study conducted by Hong et al16 who found that 29% of the plaque volume consisted of necrotic core. Although they studied both patients with acute coronary syndromes and patients with stable angina pectoris, the results for moderate-dose rosuvastatin-treated patients are in accordance with the present study, where a significant reduction in necrotic core and an increase in fibro-fatty tissue volume were found. In both studies the rosuvastatin therapy resulted in a 50% LDL reduction and the baseline LDL values were comparable on 3.0 mmol/l. In the study by Hong et al16 the changes in plaque components were not statistically significant in the low-dose simvastatin-treated group, which is also comparable to the changes in necrotic core in the low-dose rosuvastatin group. In contrast to these results with reduction in necrotic core volume, a study by Nasu et al17 showed different results after assessment of the influence of fluvastatin (60 mg/day) on plaque components in patients with stable angina pectoris. In these patients, fluvastatin therapy was accompanied by a significant increase in fibrous tissue and a significant decrease in fibro-fatty tissue, whereas the volume of necrotic core was not influenced. Compared to our study, the fluvastatin-treated patients studied by Nasu et al17 had stable angina and higher LDL-cholesterol at baseline (3.7 mmol/l), which besides the statin may have an influence on the changes in plaque components. The study by Nasu et al17 showed only 8.3% necrotic core and 24.5% fibro-fatty content which is the opposite of our results in STEMI patients, and statins may act differently depending on the plaque composition at baseline. Also, besides the clinical presentation, the two study populations differ in risk factors, as 25% of the patients studied by Nasu had diabetes compared to 5% in our study, and the medication at the enrolment time differed with more than 40% of the patients treated with either angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors in the study by Nasu. In a third study, Toi et al18 also reported a reduction in fibro-fatty volume index as the results after assessment of the influence of pitavastatin 2 mg/day versus atorvastatin 10 mg/day on plaque components in statin-naive patients with ACS. This reduction in fibro-fatty content was only seen in pitavastatin and not in atorvastatin-treated patients despite a similar LDL reduction in both groups, and no significant changes were observed in the necrotic core volume. The observation time was short, as the mean time from baseline to follow-up was 17.5 days. More than 75% of the patients were treated with an angiotensin II-receptor blocker and more than half of these patients were diabetics. Recently an IVUS-VH study has shown that non-obstructive coronary artery lesions of diabetic patients have distinct compositional and morphological features compared to non-diabetic patients21.
In the Integrated Biomarker and Imaging Study-219 a lipoprotein-associated phospholipase A2 inhibitor (darapladip) or placebo was added to a high level of standard-of-care treatment, where baseline LDL was 2.3 mmol/l. Half of the patients had ACS, and the treatment duration was one year. In this study the addition of darapladip to standard statin therapy resulted in prevention of necrotic core expansion compared to the control group who, despite statin therapy, had a progression of the necrotic core content without any significant changes in plaque volume. With IVUS-VH the influence of statin and other plaque-modifying agents can be studied, and the consequences of potential changes in plaque components can be addressed in a long-term follow-up to assess the clinical implications.
Study limitations
This study was conducted in relatively few patients and should be repeated on a larger scale to confirm the results. Also, the clinical consequences of changes in plaque composition are not known. We studied a non-culprit lesion which may not represent the plaque composition in the infarct-related artery with the STEMI event leading the patients to enrolment in the study, although a relatively high proportion of necrotic core content was found in the study lesion in a non-infarct-related coronary artery. Recently, limitations to plaque characterisation using IVUS-VH in porcine models have been published22 and confirmed by Thim et al23. This is in contrast to the findings of the PROSPECT study24 where examination of human coronary arteries revealed a connection between the NC content and the clinical outcome of the patients examined. Furthermore, in a recent paper the reproducibility of virtual histology plaque classification has been examined and it was found that there was a variation both between local analysts and more particularly between local and core-laboratory centres25. This needs to be taken into account when comparing data from different centres.
Conclusion
In statin-naive patients with STEMI, rosuvastatin therapy for 12 months resulted in a significant reduction in LDL-cholesterol accompanied by plaque regression and reduction in necrotic core volume. However, a significant decrease in necrotic core volume was only seen in the high-dose rosuvastatin group.
Acknowledgements
We would like to express our appreciation to the personnel at the catheterisation laboratory, Odense University Hospital, for their involvement in the study. We would also like to thank study nurse Helle Cappelen for her involvement in this study.
Conflict of interest statement
H. S. Hansen is a member of the Advisory Board and has received consultant fees from AstraZeneca. A. Junker is a member of the Advisory Board and has given lectures at AstraZeneca meetings. The other authors have no conflicts of interest to declare.

