Abstract
Aims: Catheter-based intravascular near-infrared spectroscopy (NIRS) detects a lipid signal from atherosclerotic plaque. The aim of this study was to describe the effect of carotid artery stenting (CAS) on the lipid signal in a carotid stenosis.
Methods and results: We performed NIRS combined with intravascular ultrasound (IVUS) during 120 CAS procedures. Minimal luminal area (MLA) and plaque burden (PB) at the site of MLA were measured with IVUS and lipid core burden index (LCBI), maximal LCBI in a 4 mm segment of the artery (LCBImax) and LCBI in a 4 mm segment at the site of MLA (LCBImla) with NIRS-derived chemograms. NIRS-IVUS imaging was performed at baseline, after stent implantation and after balloon post-dilatation. The most common lesion type was the fibrocalcific plaque (76%). Lipid-rich plaque (LCBImax ≥400) was present in 33% of carotid stenoses and in 20% at the site of MLA. Median MLA increased significantly from baseline to stent implantation (3.63 mm2 to 5.56 mm2, p<0.001) and to post-dilatation (5.56 mm2 to 12.03 mm2, p<0.001). Median LCBI, LCBImax and LCBImla significantly decreased from baseline to stent implantation: LCBI (60 to 8, p<0.001), LCBImax (294 to 60, p<0.001) and LCBImla (124 to 0, p<0.001). Post-dilatation of the stent had no further significant effect on median LCBI (8 to 5, p=0.890), LCBImax (60 to 50, p=0.690) and LCBImla (0 to 0, p=0.438).
Conclusions: Carotid artery stenting significantly reduced the NIRS-derived lipid core burden index at the stented segment.
Introduction
Advances in invasive and non-invasive vascular imaging have led to better understanding of the pathophysiology of atherosclerosis. However, most of the in vivo studies were performed on coronary arteries. Catheter-based intravascular near-infrared spectroscopy (NIRS) is a relatively novel imaging method to detect a lipid signal from an atherosclerotic plaque1,2. The accuracy of NIRS to detect lipid core-containing atherosclerotic plaques has been validated in coronary autopsy specimens1 and in vivo2. NIRS has been utilised to assess coronary atherosclerosis and the effect of various interventions on the lipid signal in coronary plaques3,4,5. Studies with NIRS have demonstrated that the presence of lipid-rich coronary plaques in patients undergoing percutaneous coronary intervention (PCI) is related to the incidence of cardiovascular events during long-term follow-up6,7, the risk of periprocedural myocardial infarction during PCI8,9,10, and presentation with stable angina versus acute coronary syndrome11,12. They further described the coronary artery response to PCI as redistribution and reduction of the lipid core in the stented segment13,14,15. A similar response to stent implantation (i.e., reduction of the lipid signal) could be expected in other vascular territories. We have already reported the safety and feasibility of the NIRS-IVUS imaging of carotid arteries in vivo and the distribution of LCBI in carotid stenosis16 and improved characterisation of the composition of carotid stenosis with multimodality imaging including NIRS17.
The aim of this study was to describe the effect of carotid artery stenting (CAS) on the lipid signal in a carotid stenosis. The response of the lipid signal was assessed with intravascular NIRS-derived lipid core burden index (LCBI) in a segment of common and internal carotid artery before and after stent implantation.
Methods
DESIGN
In Motol University Hospital, Prague, Czech Republic, we prospectively enrolled patients undergoing CAS who met the eligibility criteria into the registry. The study protocol was approved by the Motol University Hospital Ethics Committee. All patients provided written informed consent for their participation. Imaging with NIRS-IVUS was performed prior to stent implantation (baseline), after stent implantation and after balloon post-dilatation.
STUDY POPULATION
Patients were referred to CAS by a neurologist, cardiologist or vascular surgeon based on Doppler ultrasound and/or CT angiography imaging. Doppler ultrasound was available in 104 (87%) and CT angiography in 51 (43%) carotid stenoses. Stenoses were quantified angiographically according to the North American Symptomatic Carotid Endarterectomy Trial Collaborators criteria18. Inclusion criteria were symptomatic (≥50%) or asymptomatic (≥70%) stenosis of the internal carotid artery (ICA) in a patient who was considered eligible for CAS with a protection device. Eligibility criteria were in accordance with the ESC Guidelines on the diagnosis and treatment of peripheral artery diseases19. Exclusion criteria were prior ipsilateral CAS, advanced heart failure, renal insufficiency with an eGFR ≤30 mL/min/1.73 m2, active bleeding, history of intracranial bleeding, imaging evidence of intraluminal thrombus, lesions with angiographic string sign or near occlusion and where the carotid anatomy was deemed inappropriate for NIRS-IVUS imaging (kinking, severe tortuosity). Carotid stenosis was considered symptomatic if the patient suffered from stroke, transient ischaemic attack or amaurosis fugax ipsilateral to the stenosis in the previous six months.
CAROTID STENTING
The CAS procedures were all performed by an experienced operator20,21. Procedures were performed using a 7 or 8 Fr guiding catheter or a long 6 Fr sheath. The antithrombotic regimen included administration of 100 mg of aspirin and 300 mg of clopidogrel before CAS; a bolus of heparin (70 IU/kg) was administered at the beginning of CAS. Types and manufacturers of the embolic protection devices and stents are listed in Supplementary Table 1. Use of an embolic protection device was mandatory prior to NIRS-IVUS imaging. Stents were post-dilatated in 98% of the procedures with a 4-5 mm angioplasty balloon up to 8 atmospheres.
NEAR-INFRARED SPECTROSCOPY AND INTRAVASCULAR ULTRASOUND IMAGING TECHNIQUE
NIRS and IVUS were performed utilising a single 3.2 Fr rapid-exchange catheter TVC Insight™ Imaging System™ (Infraredx, Burlington, MA, USA). The system utilises absorbance and backscattering of the near-infrared wavelength to determine chemical composition1. The result of NIRS examination is presented as a chemogram – a two-dimensional map with the x-axis indicating the position of the measurement on the long axis of the vessel and the y-axis circumferential position of the measurement on the cross-section through the vessel – where every picture element (pixel) represents one spectroscopic measurement. Every measurement is binary coded as yellow (positive) or red (negative). The LCBI is a summary measure of the lipid signal along the interrogated segment of the vessel on a scale of 0 to 1,000. The LCBI is calculated as a fraction of yellow pixels in the chemogram multiplied by a factor of 1,0001. The NIRS probe at the distal tip of the catheter acquires 40 spectroscopic measurements per second during automated pullback through the catheter at a speed of 0.5 mm/s and 16 rotations per second1. IVUS images are acquired via a 40 MHz transducer rotating at the same speed as the NIRS probe.
Three NIRS-IVUS measurements were performed during one CAS procedure (Figure 1). Ten lesions (8%) required predilatation with a 2.0 mm balloon to facilitate passage of the catheter. The first pullback was performed prior to stent implantation (i.e., at baseline). The NIRS-IVUS catheter was advanced over a 0.014” guidewire into the ICA distal to the stenosis, and pullback was performed through the stenosis into the common carotid artery (CCA). The region of interest (ROI) was defined from IVUS as a 40 mm segment of the ICA and CCA corresponding to the landing zone of the stent. The second pullback was performed after stent implantation from the distal to the proximal edge of the stent. The third pullback was performed after balloon post-dilatation of the stent.
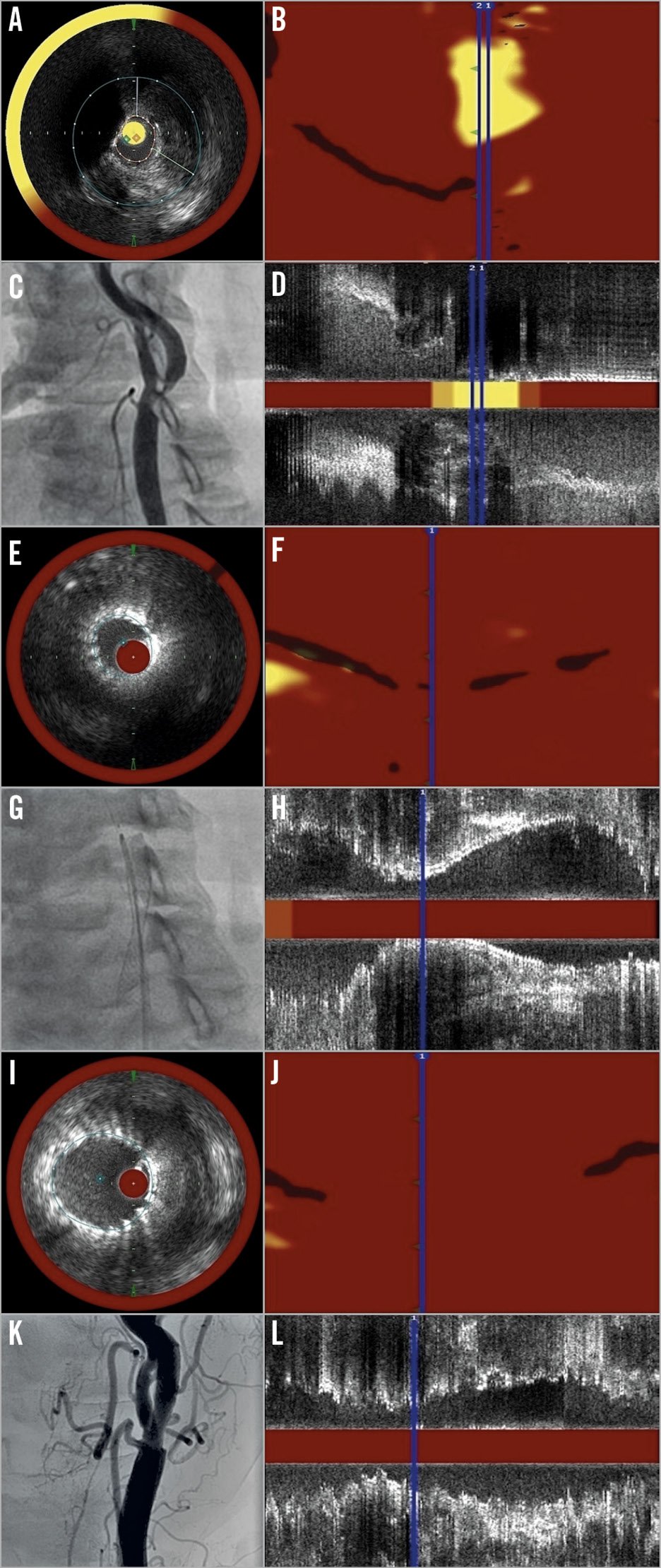
Figure 1. A panel of pictures demonstrating the imaging process in the study. A) – D) Imaging at baseline. E) – H) Imaging after stenting. I) – L) Imaging after balloon post-dilatation. A), E) & I) Cross-section NIRS-IVUS frames at the site of minimal lumen area. B), F) & J) Chemograms. C), G) & K) Angiograms of carotid bifurcation. D), H) & L) Corresponding longitudinal NIRS-IVUS images.
INTRAVASCULAR ULTRASOUND ANALYSIS AND DEFINITIONS
Off-line quantitative greyscale IVUS analysis was performed using the QIvus™ software (Medis, Leiden, the Netherlands). The definitions of the IVUS measurements are reported in the expert consensus document22 (Supplementary Appendix 1). IVUS was used to assess minimal luminal area (MLA), plaque burden (PB) and calcifications. Plaques at the site of MLA were defined as fibrocalcific if any calcifications were present and fibrous if there were no calcifications.
NEAR-INFRARED SPECTROSCOPY ANALYSIS AND DEFINITIONS
Off-line quantitative and qualitative analysis of NIRS-derived chemograms in the ROI was performed using the QIvus software (Medis). Chemograms were available in all 120 cases at baseline, in 114 cases (94%) after stent implantation and in 114 cases (94%) after post-dilatation. At least two chemograms were available in all patients. The following parameters were used to quantify the lipid signal from the ROI: LCBI, maximal LCBI in any 4 mm segment of the artery (LCBImax) and LCBI in the 4 mm segment at the site of minimal luminal area (LCBImla), defined as the segment 2 mm proximal to 2 mm distal from the site of MLA. A lipid-rich plaque (LRP) was defined as a minimum 4 mm segment on the chemogram with LCBImax ≥400 as previously described7,8,19. Lipid core shift was defined as a new lipid signal (yellow spot) in a minimum 2 mm segment of a chemogram (yellow 2 mm block chemogram) which was free of the lipid signal (red 2 mm block chemogram) on the previous chemogram.
STATISTICAL ANALYSIS
Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA). Normally distributed continuous variables are presented as mean±standard deviation (SD) and compared with the Student’s t-test. Non-parametric continuous variables are presented as median and interquartile range (IQR) and compared with a Wilcoxon matched-pair signed-rank test for paired samples or a Mann-Whitney U test for independent samples. Categorical variables are presented as frequency (%) and compared with Pearson’s χ2 test. A linear association between continuous variables is expressed with correlation plots and Pearson’s correlation coefficient (r). A two-sided p-value of 0.05 or less was considered to indicate statistical significance.
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
Between April 2013 and June 2017, 159 patients underwent 173 CAS procedures at the study institution. Periprocedural NIRS-IVUS imaging was performed in 120 CAS procedures in 112 patients (Table 1). The mean patient age was 67±8 years and two thirds were male. There was a high prevalence of hypertension (87%), coronary artery disease (50%), smoking (41%) and diabetes (37%). Two thirds of patients were considered to be high-risk for surgery. The procedural characteristics are listed in Table 2. Technical success of CAS (residual stenosis less than 30%) was achieved in all patients. All 120 (100%) patients underwent a 30-day follow-up. Two (1.7%) periprocedural TIAs occurred. During the 30-day follow-up two (1.7%) patients suffered from an ipsilateral stroke and there were no fatal events.
NEAR-INFRARED SPECTROSCOPY AND INTRAVASCULAR ULTRASOUND ASSESSMENT OF CAROTID STENOSIS
NIRS-IVUS provided several detailed insights into carotid stenosis (Table 3). Fibrocalcific and lipid-rich plaques at the site of MLA were present in 76% and 20% of cases, respectively. LRP in the whole ROI was identified in 33% of cases. Although baseline median LCBImax was significantly higher than median LCBImla (294 [157-449] vs. 124 [0-338], p<0.001), plaque burden (PB) at the site of MLA was significantly larger than PB at the site of LCBImax (PBmla 88.5±5.2% vs. PBlcbimax 63.7±20.0%, p<0.001). We constructed correlation plots for IVUS-derived measurements and LCBI (Figure 2). There was only a weak linear correlation between MLA and LCBImla (r=-0.183, p=0.045) and no correlation between PB and LCBImla (r=0.104, p=0.433). Median LCBI, LCBImax and LCBImla did not differ significantly between sexes, symptomatic or asymptomatic stenoses, presence or absence of coronary artery disease and statin treatment status (Supplementary Table 2).
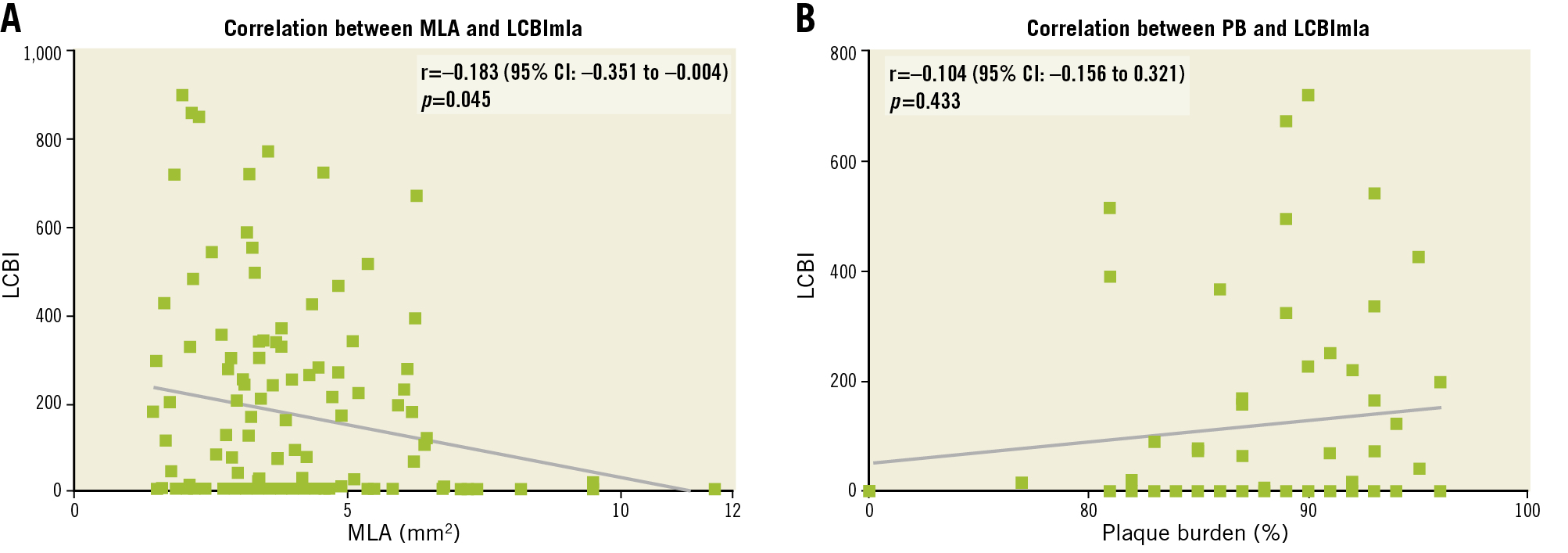
Figure 2. Correlation plots with Pearson’s correlation coefficient (r). A) Correlation between LCBImla and minimal luminal area. B) Correlation between LCBImla and plaque burden.
CHANGE IN NEAR-INFRARED SPECTROSCOPY-DERIVED LIPID CORE SIZE DURING CAROTID STENTING
Median MLA increased significantly from baseline to stent implantation (3.63 mm2 to 5.56 mm2, p<0.001) and from stent implantation to post-dilatation (5.56 mm2 to 12.03 mm2, p<0.001) (Figure 3A). Median (IQR) LCBI (Figure 3B), LCBImax (Figure 3C) and LCBImla (Figure 3D) decreased significantly from baseline to stent implantation: LCBI (60 [26-116] to 8 [1-33], p<0.001), LCBImax (294 [157-449] to 60 [9-217], p<0.001) and LCBImla (124 [0-338] to 0 [0-2], p<0.001). Post-dilatation of the stent had no further significant effect on median LCBI (8 [1-33] to 5 [0-38], p=0.890), LCBImax (60 [9-217] to 50 [0-224], p=0.690) and LCBImla (0 [0-2] to 0 [0-0], p=0.438). No significant difference in LCBI was observed after stenting with an open-cell stent versus a closed- or hybrid-cell stent (Supplementary Table 3). Lipid core shift was observed in 16 (13%) cases after stent implantation and in 27 (23%) cases after post-dilatation.
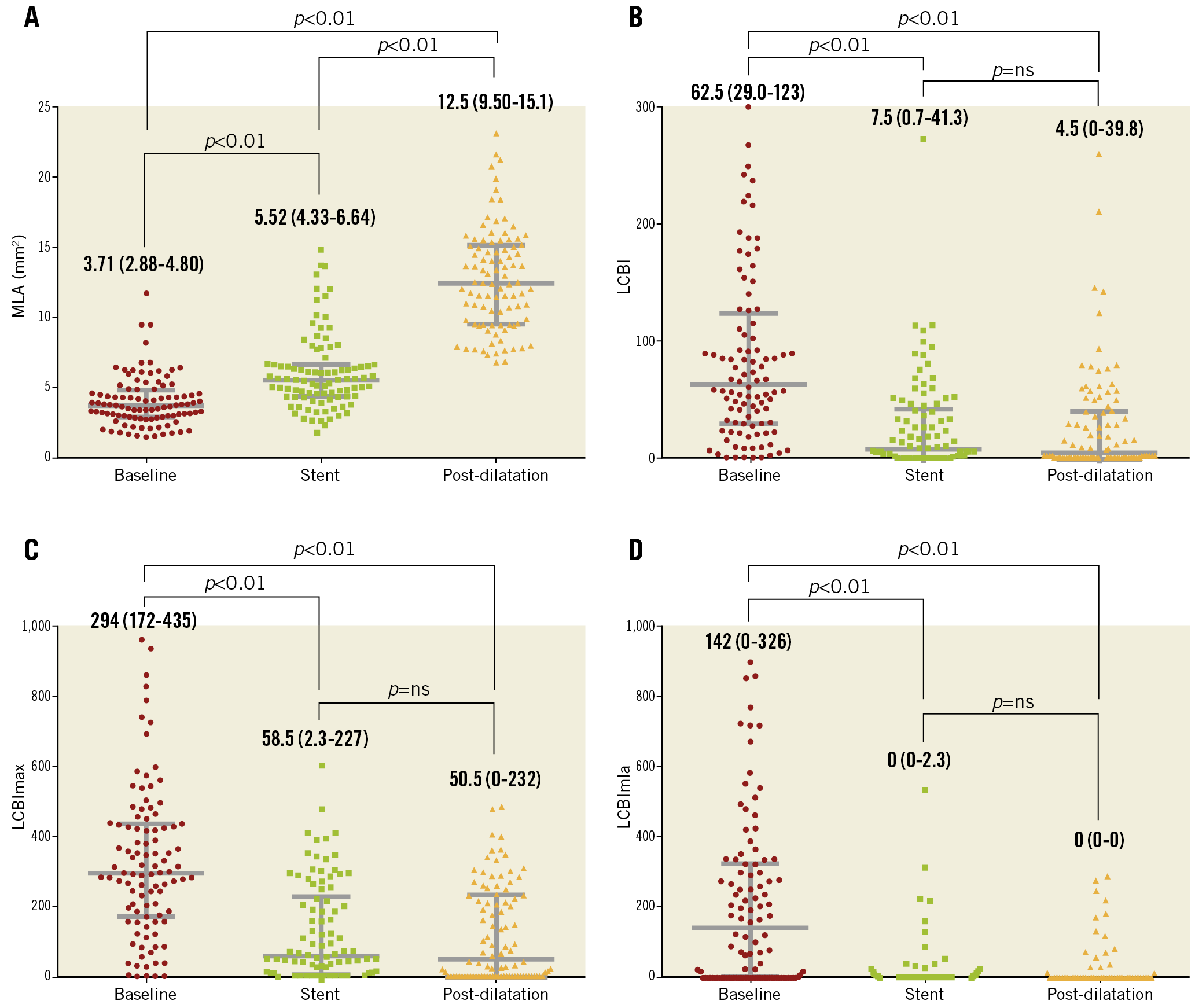
Figure 3. Scatter plots with median and IQR bars of A) minimal luminal area, B) lipid core burden index (LCBI), C) maximal lipid core burden index in any 4 mm segment of the artery (LCBImax) and D) lipid core burden index in the 4 mm segment at the site of minimal luminal area (LCBImla) at baseline, after implantation of the stent and after post-dilatation of the stent.
Discussion
The present study was the first prospective study to use serial NIRS-IVUS imaging in patients undergoing CAS to investigate lumen, plaque and lipid core changes caused by stent implantation and balloon post-dilatation. The major findings are summarised as follows. Plaques from severe carotid stenosis were mostly fibrocalcific but one third also contained lipid-rich regions. These LRP were at sites with a high plaque burden. Implantation of a self-expanding carotid stent, although resulting only in modest luminal expansion, led to a significant decrease in the LCBI. On the other hand, post-dilatation of the stent with an angioplasty balloon caused much greater lumen expansion but did not result in any further significant change in LCBI and was associated with lipid core shift in 23% of cases.
Recently published studies reported favourable long-term outcomes of both endarterectomy and stenting of carotid stenosis23,24. Whereas endarterectomy restores the lumen diameter and removes the plaque, stenting restores the lumen by pushing the plaque outwards and, because it cannot be compressed, distending the media and external elastic membrane. The novel finding from our study is that CAS not only dilates the lumen but also significantly reduces the lipid signal. Whether this reduction also means long-term stabilisation after healing of the stent with neointima, hence the comparable long-term clinical effect of the two fundamentally different procedures – stenting and endarterectomy – requires further study. We assume that several mechanisms could have contributed to the observed reduction of the LCBI after stent implantation.
The stent disrupts the fibrous cap and cuts into the soft lipid core, causing embolisation of the semi-liquid content. This might be the “culprit” in most ischaemic strokes that occur during or within hours to days after CAS, either by embolisation of the lipid core or by protrusion of the lipid-rich plaque through stent struts followed by thrombosis on the disrupted plaque and delayed embolisation. This mechanism was proposed for lipid-rich plaque-related myocardial infarction after PCI8,9,10 and was also observed with an optical coherence tomography (OCT) imaging during CAS25,26. The limited size of our study and low incidence of adverse clinical events do not allow us to draw any conclusions regarding lipid core embolisation.
The stent causes compression and shift of the lipid core within the plaque. Lumen expansion increases the distance of the catheter from the lipid core, thus attenuating the lipid signal which would not be actual lipid core reduction. Lipid core shift was observed in 23% of cases after post-dilatation as new yellow spots in segments that were without any lipid signal on the baseline chemogram.
The stent reflects near-infrared light obscuring the signal from the lipid core compressed under it. When the stent is not fully expanded, the struts are very dense and cover a significant proportion of the luminal area, creating a mirror to the near-infrared light beam. This would again lead to decreased LCBI, which would not be actual reduction of the lipid core. Post-dilatation decreases the density of the stent struts, which could make the lipid core compressed under the stent reappear on NIRS. Some cases of lipid core shift when there was major reduction of LCBI after stent implantation and some increase after post-dilatation could be explained by this mechanism.
Limitations
The present study is not without inherent limitations, mainly due to the in vivo setting. First, we did not routinely use any imaging method to detect silent microembolisation during CAS or new ischaemic lesions in the brain, which could be attributed to lipid core embolisation. Second, detection of lipid core with NIRS was not compared with another imaging method, as there is no gold standard for plaque composition in vivo. Nevertheless, in an appropriately sized lumen, there is no reason to expect NIRS signals to differ between the carotid and coronary arteries. In our study, the mean minimal luminal diameter of the ICA was 1.94±0.42 mm, and the mean reference luminal diameter of the ICA was 4.34±0.82 mm. The manufacturer of the TVC Imaging System reports a maximal imaging depth of 10 mm for IVUS and 3.5 mm for NIRS1,2, assuming that the imaging depth should be sufficient for stenosis of the ICA.
Conclusions
Carotid artery stenting significantly reduced the NIRS-derived lipid core burden index at the stented segment. During CAS, a significant reduction of LCBI was observed after stent implantation, whereas post-dilatation of the stent had no further significant effect on LCBI.
|
Impact on daily practice NIRS-IVUS assessment of the carotid stenosis in patients undergoing CAS provides additional morphological and compositional information that might prove useful in tailoring the treatment strategy. The best treatment option (specifically designed stents, aggressive antithrombotic and lipid-lowering therapy or endarterectomy) for lipid-rich carotid plaques needs to be determined in future trials. |
Funding
This work was supported by the Ministry of Health, Czech Republic – conceptual development of research organisation, Motol University Hospital, Prague, Czech Republic. Grant number SVV-2013-266509 from the Charles University in Prague.
Conflict of interest statement
The authors have no conflicts of interest to declare.

