
Abstract
Aims: There is evidence for a lower mortality in female patients after transcatheter aortic valve implantation (TAVI), but the underlying reasons for the gender-specific differences in prognosis are not well understood. In particular, the role of left ventricular dysfunction is unclear. In this study, we addressed the question of whether differences in left ventricular ejection fraction (LVEF) could account for the gender benefit for female TAVI patients.
Methods and results: From January 2011 to December 2013, a total of 15,616 patients treated with TAVI were prospectively enrolled in the German Aortic Valve Registry (GARY). For the present analysis, female TAVI patients (N=8,456) were compared with male TAVI patients (N=7,160) with a particular focus on LVEF. Mortality at one year was 18.1% in women and 22.6% in men (p<0.001). Multivariate analysis also revealed that female gender was associated with a lower one-year mortality (adjusted hazard ratio [HR] 0.88 [0.81-0.95]). There was no difference in gender-specific mortality in patients with baseline LVEF >50% (women: 16.4%; men 17.6%, p=0.268), but in patients with LVEF 30%-50% (21.0% versus 25.7%, p<0.001) and <30% (26.2% versus 37.6%, p<0.001) one-year mortality was significantly lower in women than in men.
Conclusions: The survival benefit for women over men after TAVI was only observed in patients with a preprocedural LVEF ≤50%.
Abbreviations
AS: aortic stenosis
BMI: body mass index
COPD: chronic obstructive pulmonary disease
GARY: German Aortic Valve Registry
IQR: interquartile range
LVEF: left ventricular ejection fraction
NYHA: New York Heart Association
PAD: peripheral artery disease
PCI: percutaneous coronary intervention
SAVR: surgical aortic valve replacement
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
Introduction
Following continuous advances and improvements over the last ten years, TAVI has become the standard of care for inoperable patients and is becoming increasingly common for high-risk surgical patients with severe symptomatic AS1. In daily clinical practice, women account for at least 50% of the patients treated with TAVI. There is convincing evidence for a significantly higher survival rate in female as compared with male TAVI patients2-8; however, the reasons for the gender-specific differences in outcome are not well understood9,10. A reduced LVEF is a strong independent predictor of early and midterm mortality after TAVI, and it affects men and women differently3,11-14. Furthermore, gender has been shown to influence cardiac remodelling after treatment for AS15. Nevertheless, the relationship between gender, LVEF, and prognosis in TAVI patients remains unclear.
We retrospectively evaluated gender-specific differences and one-year outcomes with regard to the LVEF within the large, contemporary GARY registry that prospectively enrolled 15,616 consecutive TAVI patients between 2011 and 2013. We addressed the question of whether differences in LVEF could account for the gender benefit for female TAVI patients.
Materials and methods
THE GERMAN AORTIC VALVE REGISTRY
GARY is a joint project of the German Cardiac Society and the German Society for Thoracic and Cardiovascular Surgery that has compiled a complete data set for both TAVI and SAVR patients across Germany16-18. It is a prospective, multicentre registry designed to monitor the efficacy and outcome of interventional and surgical aortic valve procedures. Patients undergoing invasive treatment for acquired aortic valve stenosis have been enrolled consecutively; the only exclusion criterion is refusal to participate. The registry design has been previously described in detail16. Ethics approval was obtained from all participating centres, and patients’ written, informed consent was obtained preoperatively.
Follow-up was obtained at one year (median follow-up 371 days and IQR 360-380 days) based on the medical records and on physician and patient interviews.
TAVI was classified as successful when there was adequate prosthesis position, no coronary obstruction and good function of the prosthesis.
STATISTICAL ANALYSIS
The descriptive analysis includes women and men treated with TAVI. Continuous scaled variables are given as median and interquartile range. Statistical significance was tested in a two-sided manner with the alpha level of 5%. Comparison of baseline values between the subgroups was carried out using the Mann-Whitney U test (any differences) for continuous variables. Categorical variables were analysed by Pearson’s χ2 test or Fisher’s exact test where applicable. LVEF is a categorical variable and was handled as such.
Survival curves in patients with baseline LVEF >50%, 30-50% and <30% were constructed for time-to-event variables using Kaplan-Meier estimates and compared by the log-rank test. We evaluated the effect of gender on one-year mortality in the overall TAVI population and in the transvascular and transapical groups. We also evaluated independent predictors for one-year mortality (using hazard ratios) separately in women and men. In addition to gender, the following baseline variables that had been specified by clinical considerations were first tested in univariate analysis: age (per year), BMI <22 kg/m², LVEF 30-50 and <30%, NYHA IV classification, decompensated heart failure, coronary artery disease, prior myocardial infarction, prior PCI, prior cardiac surgery, prior pacemaker implantation, prior defibrillator implantation, pulmonary hypertension, arterial hypertension, diabetes mellitus, atrial fibrillation, neurological dysfunction (Rankin ≥2), chronic obstructive pulmonary disease (COPD), peripheral artery disease (PAD), chronic renal failure, chronic renal failure requiring dialysis, aortic regurgitation ≥II°, mitral regurgitation ≥II°, severe tricuspid regurgitation, aortic valve orifice, Delta Pmax and Delta Pmean. Moreover, the following procedural and postoperative variables that had also been specified by clinical considerations were tested in univariate analysis: Heart Team approach, additional PCI, transapical access route, balloon predilatation, rapid pacing, vascular complications, major bleeding, residual aortic regurgitation ≥II°, new-onset dialysis, atrial fibrillation at discharge, new-onset pacemaker implantation, new-onset defibrillator implantation, atrioventricular block, thromboembolic event, cerebral embolisation, sepsis, endocarditis, procedure duration, radiation time, contrast dye, aortic dissection, annular rupture, cardiac tamponade, conversion to sternotomy and LV decompensation. All variables with values of p<0.05 in univariate analysis were included in the multivariate Cox regression model performed by Wald backward approach. Furthermore, testing for interaction between gender and LVEF (dichotomised) was performed as described by Heinze et al19.
All statistical analyses were performed using the SPSS statistical package, Version 19.0.0 (IBM Corp., Armonk, NY, USA). Data management and statistical analyses were performed by the BQS Institute for Quality and Patient Safety, Dusseldorf, Germany.
Results
STUDY POPULATION
In the years 2011-2013, 15,616 patients were treated with TAVI. For the present analysis, female TAVI patients (N=8,456) were compared with male TAVI patients (N=7,160). The baseline characteristics of the patients are shown in Table 1. Women treated with TAVI were older. The incidence of reduced systolic left ventricular function and coronary artery disease was lower among women as was the incidence of non-cardiac comorbidities. Women had significantly higher pressure gradients. Severe mitral and tricuspid regurgitation was more prevalent among women. The logistic EuroSCORE showed a tendency to be higher among women and the German Aortic Valve score and the STS score were significantly higher among women.
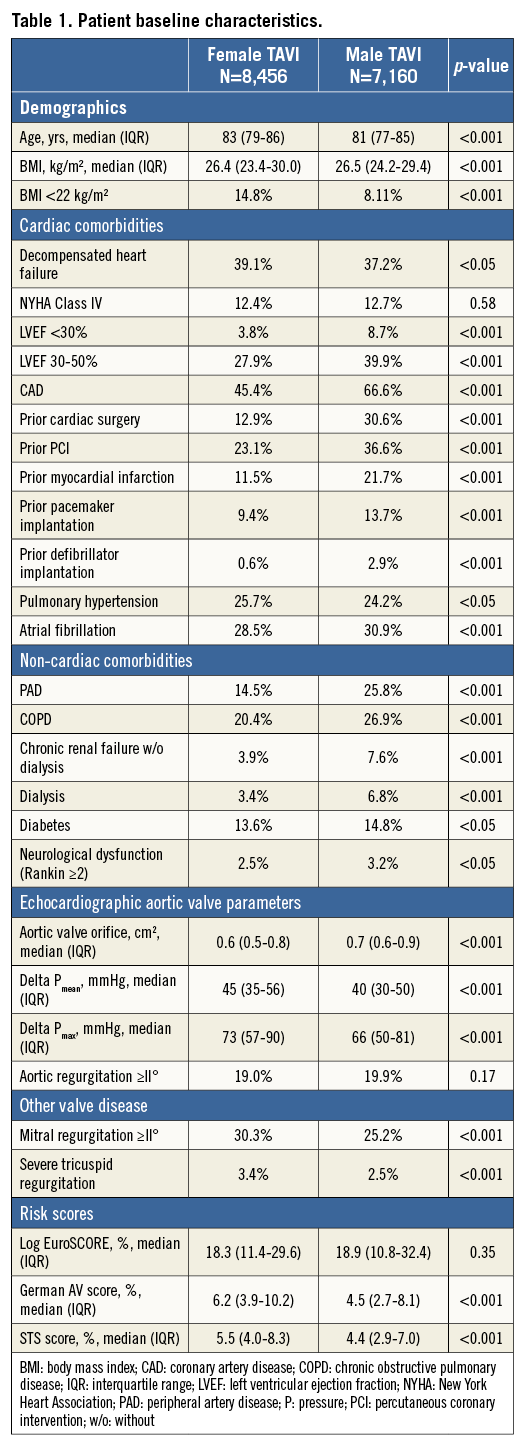
PROCEDURAL DATA
A transvascular approach was used more often among women (76.0% versus 68.2%, p<0.0001), whereas men were treated more frequently via the transapical approach (31.8% versus 24.0%, p<0.0001) (Table 2). Procedural success was high in both cohorts (women: 97.4%; men 97.3%). An additional PCI during the same procedure was performed only in a small minority of patients (women: 2.1%; men 2.1%).
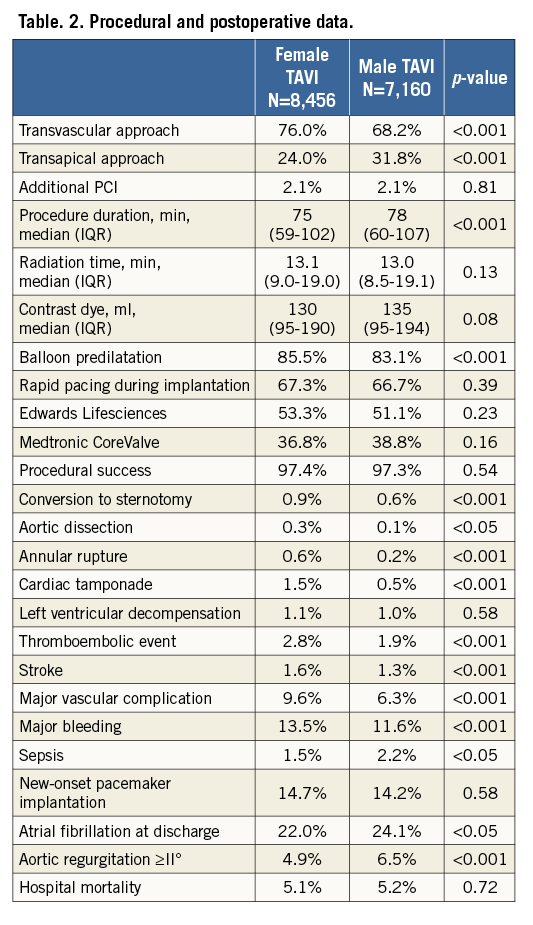
HOSPITAL OUTCOME
Hospital mortality was similar in women (5.1%) and men (5.2%). Aortic regurgitation (AR) ≥II° occurred more often in men (6.5% versus 4.9%, p<0.0001), whereas female TAVI patients suffered more often from vascular complications (9.6% versus 6.3%, p<0.0001) or major bleeding (13.5% versus 11.6%, p<0.0001) (Table 2).
ONE-YEAR MORTALITY
Mortality at one year was 18.1% in women and 22.6% in men (p<0.0001). In the multivariate analysis, female gender was associated with a lower one-year mortality after TAVI (adjusted hazard ratio [HR] 0.88 [0.81-0.95]).
ONE-YEAR MORTALITY IN RELATION TO LVEF
There was no difference in gender-specific mortality in patients with baseline LVEF >50% (women: 16.4%; men 17.6%, p=0.27) (Figure 1), but in patients with LVEF 30-50% (21.0% versus 25.7%, p<0.001) (Figure 2) and <30% (26.2% versus 37.6%, p<0.001) (Figure 3) one-year mortality was significantly higher in men than in women. In patients with LVEF <30% and with prior myocardial infarction, one-year mortality (women: 43.7%; men 40.6%, p=0.667) was similar, whereas in patients with LVEF <30% and without prior myocardial infarction one-year mortality (women: 21.4%; men 35.8%, p<0.001) was significantly lower in women.
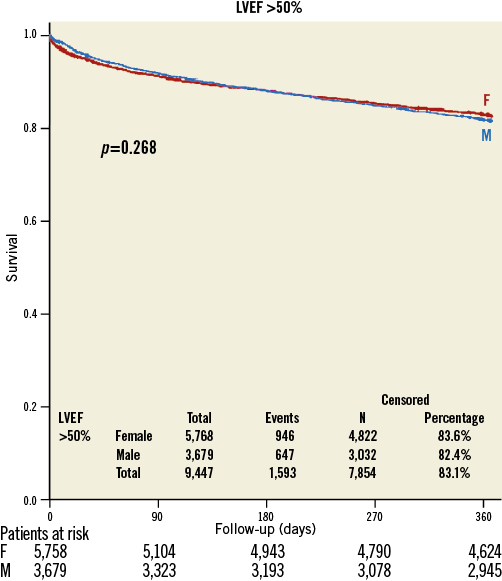
Figure 1. Kaplan-Meier survival curve in women (red) and men (blue) with preserved preprocedural systolic ejection fraction (>50%).
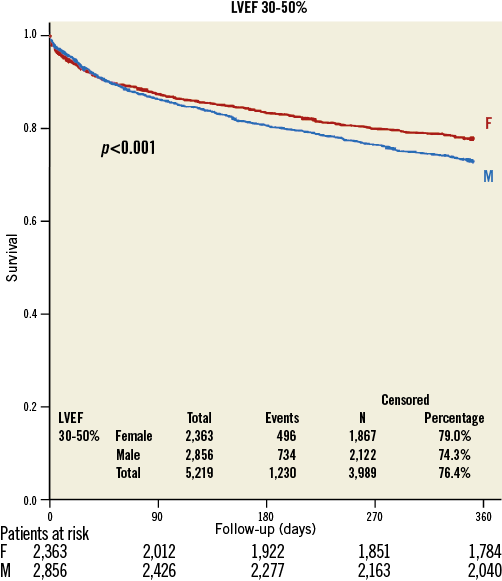
Figure 2. Kaplan-Meier survival curve in women (red) and men (blue) with moderately reduced preprocedural systolic ejection fraction (30-50%).
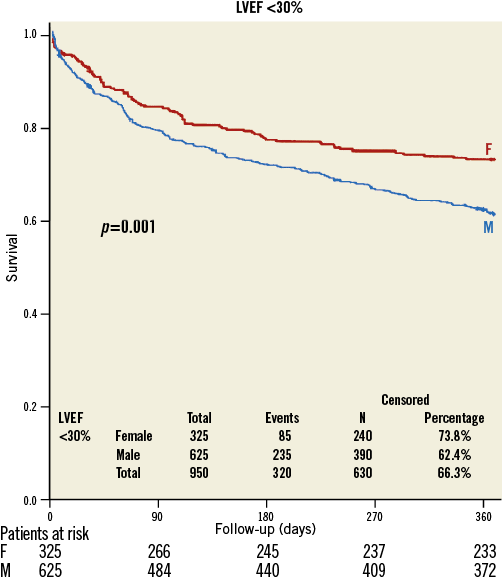
Figure 3. Kaplan-Meier survival curve in women (red) and men (blue) with severely reduced preprocedural systolic ejection fraction (<30%).
INDEPENDENT PREDICTORS FOR ONE-YEAR MORTALITY
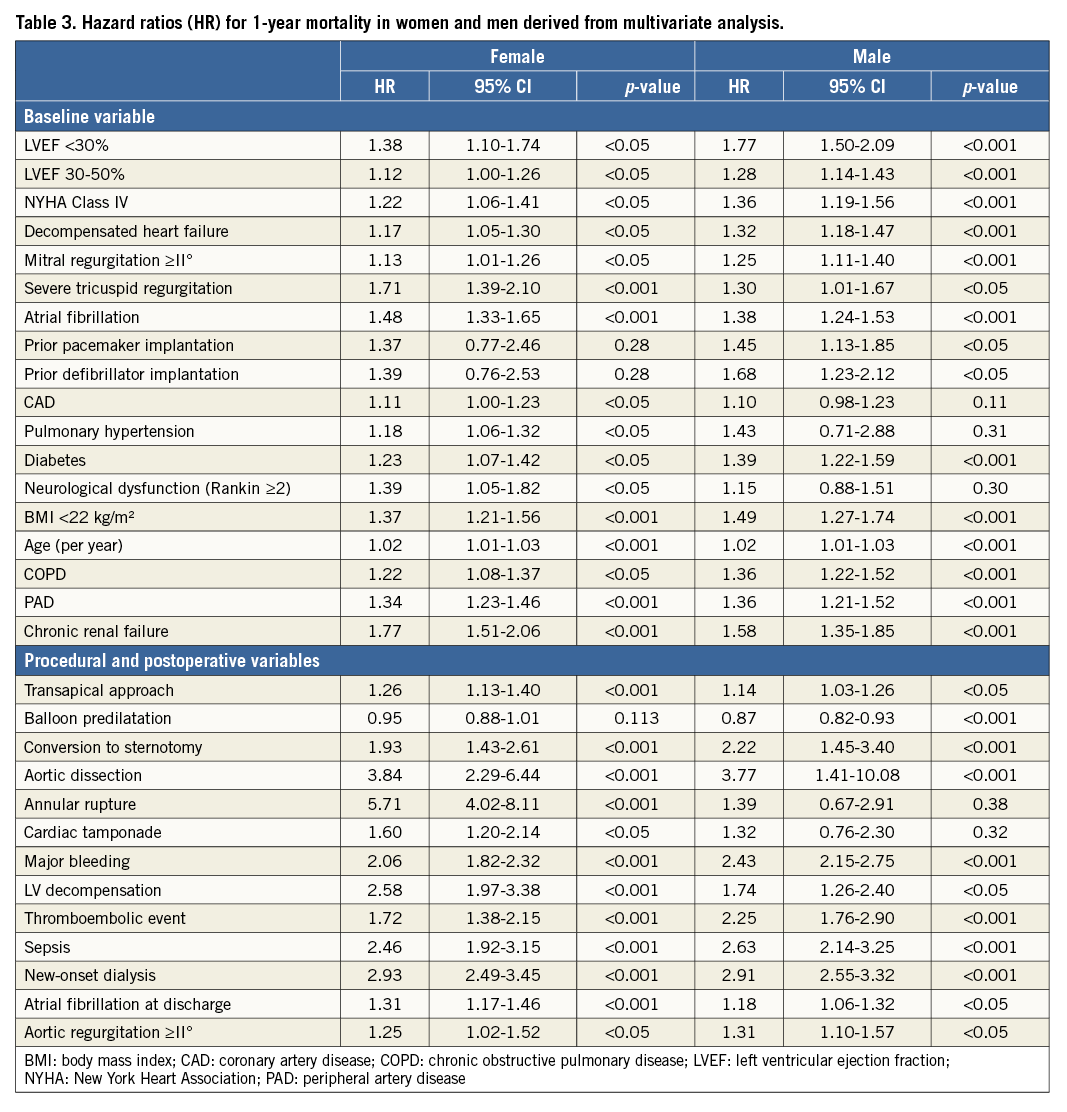
Multivariate logistic regression analysis determined the independent predictors for one-year mortality in women and men (Table 3). After adjustment for baseline, procedural and postoperative variables, LVEF <30% (women: HR 1.38 [1.10-1.74]; men: HR 1.77 [1.50-2.09]) and 30-50% (women: HR 1.12 [1.00-1.26]; men: HR 1.28 [1.14-1.43]) were associated with higher one-year mortality.
TESTING FOR INTERACTION IN THE OVERALL TAVI POPULATION
The Cox regression analysis demonstrated statistically significant interaction between gender and LVEF <30% (adjusted HR 1.37 [1.06-1.77]) and 30-50% (adjusted HR 1.23 [1.09-1.39]). The negative effect of reduced LVEF (<30% and 30-50%) on one-year mortality was more pronounced in men than in women.
TESTING FOR INTERACTION IN THE TRANSVASCULAR GROUP
The Cox regression analysis demonstrated statistically significant interaction between gender and LVEF 30-50% (adjusted HR 1.23 [1.09-1.39]). In patients with LVEF <30%, testing showed no significant interaction (adjusted HR 1.20 [0.89-1.62]).
TRANSAPICAL GROUP
In patients with transapical TAVI, female gender was not independently associated with one-year mortality. However, in patients with LVEF <30%, one-year mortality tended to be higher among men (40.0% versus 27.1%, p=0.08).
CORONARY EVENTS AT ONE-YEAR FOLLOW-UP
The rate of myocardial infarction (women: 0.2%; men 0.4%), PCI (women: 0.6%; men 0.9%), and coronary artery bypass grafting (women: 0.2%; men 0.2%) was low in both groups.
Discussion
We analysed gender-specific differences in mortality in relation to LVEF in this study of a large TAVI cohort with over 15,616 patients from the contemporary German Aortic Valve Registry. The results demonstrate that the survival benefit for women over men after TAVI can only be seen in patients with reduced baseline left ventricular function.
Concomitant impairment of LVEF is a frequent finding in TAVI patients3,5. In GARY, almost 40% of male TAVI patients presented with reduced LVEF, whereas among women the rate was about 28%. In agreement with most previously published data, other clinical characteristics of women and men treated with TAVI also differed substantially3,5. Coronary artery disease and non-cardiac comorbidities were more frequent among men. Nevertheless, the risk scores were higher among women. This is mainly due to the fact that female TAVI patients were two years older than their male counterparts and female gender itself is considered a risk factor in the logistic EuroSCORE, the STS score and the German Aortic Valve score. However, the paradox of this issue is that survival rates in female as compared with male TAVI patients are higher in most studies and female gender should not be considered a risk factor. The access route was also different between the two cohorts. In contrast to older studies, the transvascular approach was more often used among women in GARY5, which can probably be explained by the smaller sheath sizes used nowadays.
Hospital mortality was almost identical in men and women after TAVI. After discharge, however, the Kaplan-Meier survival curves begin to diverge. At one year the absolute mortality rate is over 4% higher in men. We also calculated the adjusted one-year mortality rates. In the multivariate analysis female gender was also associated with a survival benefit. The relative risk of mortality was 12% lower than in men. It has been known for some time that women have a more favourable outcome after TAVI2-8, and these results from GARY confirm these findings.
There have been several attempts to explain the gender-specific difference in mortality. First of all, men have a higher prevalence of cardiac and non-cardiac comorbidities. It is unlikely, however, that the observed survival benefit in female patients can be explained only by the difference in comorbidities. In many studies, extensive adjustments for various clinical baseline factors have failed to attenuate the gender difference3-6. Beyond that, AR after TAVI is linked to a poorer prognosis, and its rate is higher among men13,20. In agreement with previous data, post-procedural AR ≥II° occurred more often in men, but the absolute numbers did not differ substantially in this registry. In conclusion, the gender-specific difference in mortality cannot be explained solely by the higher prevalence of post-procedural AR in men. Interestingly, in patients with isolated SAVR, gender does not seem to have an impact on survival21. Women have a smaller aortic annulus than men, however, and TAVI valves demonstrate a superior haemodynamic performance compared with surgical valves in patients with a small aortic annulus22.
This study shows that the baseline LV function is a decisive factor for the gender-specific difference in mortality after TAVI. One-year mortality was significantly higher in men with LVEF 30-50 and <30% in comparison with women with LVEF 30-50 and <30%, whereas among patients with LVEF >50% no difference in survival was observed. Testing for interaction confirms the statistically significant relationship between gender, LVEF, and prognosis in TAVI patients. This is an important finding since a substantial proportion of TAVI patients have a reduced LVEF.
Gender has been shown to influence cardiac remodelling and fibrosis in patients with severe AS15,23. Women develop a more concentric form of LV hypertrophy and less LV dilatation than men. Moreover, interstitial fibrosis is less pronounced in women. In surgical biopsies, female patients had lower levels of expression of genes coding for collagen I, III, and matrix metalloproteinase 2. Due to less fibrosis, LV hypertrophy reverses better in women after SAVR23. Apart from this, coronary artery disease is less frequent in women and LV impairment is more likely to relate to AS-induced pressure overload, which will be reversed by TAVI with a favourable influence on outcome. In our analysis, the survival benefit for women over men could only be seen in patients with reduced LVEF and without prior myocardial infarction. In a study by Stangl et al, improvement of the LVEF after TAVI was significant only in women24. Furthermore, in a gender-based analysis of TAVI patients, LVEF <30% was related to mortality only in men9. All of these findings underline the importance of LV function in patients with AS who have been treated with TAVI and might explain the better outcome in women compared with men.
Limitations
In evaluating this study one must take several limitations into account. The nature of the study is exploratory and the findings need to be interpreted with caution as confounding factors may have affected the outcome. Furthermore, we did not capture data on changes in LV function after TAVI. In some patients the exact LVEF was also missing. In these patients we only know whether the LVEF was normal (>50%), moderately (30-50%) or severely reduced (<30%).
Conclusions
Concomitant impairment of LVEF affects a relevant proportion of TAVI patients. Baseline LV function is a decisive factor for the gender-specific difference in mortality after TAVI. There is no mortality difference in the acute phase. However, a higher long-term survival rate in female over male patients was only observed in patients with LVEF ≤50% and is possibly linked to a different post-procedural cardiac remodelling.
| Impact on daily practice It has been known for some time that female patients have a better survival rate after TAVI, but the underlying reasons for the gender-specific differences in prognosis are not well understood. This analysis of GARY shows that the baseline LV function is a decisive factor for the gender-specific difference in mortality after TAVI. One-year mortality was significantly higher in men with LVEF 30-50 and <30% in comparison with women with LVEF 30-50 and <30%, whereas among patients with LVEF >50% no difference in survival was observed. |
Acknowledgements
The authors acknowledge the support of Mrs E. Schäfer, BQS Institute, for project management. The authors are also very thankful to Dr E. Martinson for proofreading and editing the paper.
Funding
The responsible body of the registry is a non-profit organisation named Deutsches Aortenklappenregister gGmbH founded by the German Cardiac Society and the German Society for Thoracic and Cardiovascular Surgery. The registry receives financial support in the form of unrestricted grants by medical device companies (Edwards Lifesciences, JenaValve Technology, Medtronic, Sorin, St. Jude Medical, Symetis S.A.).
Conflict of interest statement
H. Möllmann has received speaker fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, St. Jude Medical, and Symetis. C. Hamm has received personal fees from and serves on the advisory board of Medtronic, and has received lecture fees from Edwards Lifesciences. T. Walther has received speaker fees from Edwards Lifesciences, St. Jude Medical, and Symetis. The other authors have no conflicts of interest to declare.

