Abstract
Aims: No intraprocedural assessment is currently available to evaluate the extent of nerve ablation by renal denervation (RDN). We prospectively evaluated the association of intraprocedural reduction of renal veno-arterial norepinephrine gradient with blood pressure (BP) response after RDN.
Methods and results: In 46 consecutive RDN patients, the periprocedural norepinephrine veno-arterial difference was defined as veno-arterial norepinephrine gradient. We observed a reduction of the office systolic BP from 176±19 mmHg to 165±24 mmHg (p=0.02) at three months and 163±22 mmHg (p=0.02) at six months. The mean and maximum systolic ABP decreased by 5 mmHg (p=0.03) and 9 mmHg (p=0.02), respectively. There was a decrease of the norepinephrine RV-RA difference from pre- to post-procedural levels (median 186 pg/ml [54;466] vs. 81 pg/ml [0;182], p=0.02). OBP responders (office systolic BP reduction ≥10 mmHg) showed a greater reduction of the norepinephrine gradient compared to non-responders (–290±450 pg/ml vs. –4±106 pg/ml, p=0.01). Patients with a reduction of norepinephrine gradient in both kidneys showed the most pronounced decrease of the systolic OBP (–24±14 mmHg) compared to patients with a reduction of norepinephrine gradient in only one kidney (–7±15 mmHg) or patients without a norepinephrine reduction (–3±19 mmHg, p=0.03 vs. bilateral reduction).
Conclusions: Measuring renal norepinephrine gradient during RDN may be a method to gauge the extent of renal nerve ablation. ClinicalTrials.gov Identifier: NCT01875809 URL: http://clinicaltrials.gov/ct2/show/NCT01875809?term=NCT01875809&rank=1
Abbreviations
ABP: ambulatory blood pressure (24 hr)
BP: blood pressure
BPM: beats per minute
DBP: diastolic blood pressure
E: epinephrine
GFR: glomerular filtration rate
hr: hour
HbA1c: glycosylated haemoglobin
HPLC: high performance liquid chromatography
HTN: hypertension
NE: norepinephrine
NEartery: norepinephrine in the renal artery
NEM: norepinephrine metabolisation
NEvein: norepinephrine in the renal vein
NT-proBNP: N-terminal pro-brain natriuretic peptide
OBP: office blood pressure
RA: renal artery
RDN: renal denervation
RV: renal vein
SBP: systolic blood pressure
Introduction
Renal denervation (RDN) may represent a treatment option for resistant hypertension based on significant blood pressure (BP) reductions presented in the SYMPLICITY HTN-1 and HTN-2 trials1-3. Patients with office systolic BP ≥160 mmHg and at least three antihypertensive medications were included in these trials. An office systolic BP reduction of 20-30 mmHg at six months was documented as primary outcome, with sustained efficacy at three years1-3. The subgroup of patients receiving ambulatory blood pressure monitoring (ABPM) had a mean systolic BP reduction of approximately 10 mmHg at six months3-6. The recently published SYMPLICITY HTN-3 trial was a randomised, single-blinded, sham-controlled study of 535 patients in 88 centres in the USA. The office BP and ABPM were reduced at six months after RDN compared to baseline, but without significant differences to the sham intervention7. The SYMPLICITY HTN-3 trial and the accompanying editorial emphasise the need to identify patient and procedural characteristics serving as predictors for a significant BP reduction after RDN7,8. Several patient characteristics have already been identified, including higher pre-procedural systolic BP, higher body mass index, race, use of calcium channel blockers, and impaired cardiac baroreflex sensitivity4-6,9.
There had already been previous attempts to identify predictive procedural characteristics for renal denervation, mainly based on catecholamine assessments. Transmitter release by sympathetic nerves is the main source of norepinephrine (NE) in plasma, with the heart and the kidney having the highest veno-arterial catecholamine differences10. In the SYMPLICITY HTN-1 trial, renal NE spillover assessed by radioactive isotope dilution displayed a reduction of 47% from the RDN procedure to the follow-up performed after 15-30 days2. However, this approach did not allow a prediction of BP response based on the immediate intraprocedural changes, most likely due to the small sample size (n=10).
In our study we tested the hypothesis that the periprocedural reduction in renal veno-arterial NE gradient, based on selective renal arterial and venous measurements before and after the procedure, may reflect the extent of sympathetic denervation and predict the BP response after RDN.
Methods
STUDY POPULATION AND CLINICAL PARAMETERS
Patients aged 18-85 years with resistant hypertension were eligible for inclusion in this prospective study after documentation of a systolic BP ≥160 mmHg (average of three independent measurements while the patient was seated) (hereafter referred to as office blood pressure) under stable treatment with at least three antihypertensive medications at maximally tolerated doses including a diuretic. The exclusion criteria were renal artery stenosis, a solitary kidney, obstructive sleep apnoea, thyroid disorders, elevated cortisol levels, elevated aldosterone/renin ratio, acromegaly, elevated plasma metanephrine, anaemia, and extensive use of alcohol, drugs, extensive use of liquorice or NSAIDS. After screening 53 consecutive patients planned for the RDN procedure, three patients refused to participate in this prospective trial. Fifty patients fulfilled all inclusion criteria and none of the exclusion criteria and gave written informed consent.
In order to assess the impact of sedation and cardiac ablation on arterial NE concentrations, we additionally evaluated 20 patients undergoing electrophysiologic ablation for atrial fibrillation.
The study was conducted in accordance with the provisions of the Declaration of Helsinki and of the International Conference on Harmonization Good Clinical Practice. Protocol and informed consent approval was obtained from the medical ethics committee of Witten/Herdecke University. The study outline was registered at clinicaltrials.gov: NCT01875809.
AMBULATORY BLOOD PRESSURE MONITORING
Twenty-four hour ambulatory blood pressure (ABP) measurements (oscillometric Spacelabs 90207-32 monitor; Spacelabs Healthcare, Issaquah, WA, USA) were performed before RDN, the day after RDN and at three and six-month follow-up. Cuff size was selected according to the recommendations of the manufacturer. Measurements were taken every 20 min during the day and every 60 min during the night to reduce sleep disturbances, which might have influenced BP measurements. Maximum and mean systolic and diastolic BPs were determined for each patient. Twenty-four hour ECG assessments (Lifecard CF Life-033834 Del Mar Reynolds Medical; Spacelabs Healthcare) were performed before RDN, directly after RDN and after three and six months. The medication was documented during the entire study.
LABORATORY ASSESSMENT
We evaluated different laboratory variables in peripheral venous samples, including creatinine, cystatin C, NT-proBNP, interleukin-6, HbA1c, renin, aldosterone, aldosterone-renin ratio, and metanephrine and normetanephrine levels before RDN and at follow-up. Blood was drawn in a supine position after five minutes of rest.
RENAL DENERVATION PROCEDURE
All operators were trained for the renal denervation procedure in February 2011 and had performed at least five RDN procedures prior to treatment of the first study patient. Renal angiograms were performed via femoral access to confirm anatomic eligibility. Using a guiding catheter, the treatment catheter was introduced into each renal artery, while the sequence of the kidneys was chosen randomly to avoid bias. Bilateral RDN was performed using the single-point ablation catheter (Flex; Medtronic/Ardian, Mountain View, CA, USA) according to the manufacturer’s instructions. The number of ablation points, location, energy delivery in watts, duration in seconds, temperature rise in degrees Celsius, and the impedance drop in ohms were assessed for each ablation point independently. For each renal artery, we performed between four and eight ablations over two minutes, including all accessory renal arteries with a mean diameter ≥4 mm. We included a cranial ostial ablation point in all renal arteries.
PRE- AND POST-PROCEDURAL VENO-ARTERIAL CATECHOLAMINE SAMPLING
As specified by the study protocol and the informed consent, we placed an additional 6 Fr hockey stick guiding catheter selectively in the renal veins (Figure 1). Under constant analgosedation with propofol (0.25-0.5 mg/kg bolus followed by 3-5 mg/kg/hr infusion) and fentanyl (0.5-1 mcg/kg) for at least five minutes, we sampled 6-10 cc of arterial and venous blood at the beginning and one minute after completion of ablation on each respective kidney in blood collection tubes containing ethylenediaminetetraacetic acid (EDTA). The respective arterial and venous samples were drawn after ablation of the first renal artery and before RDN of the contralateral renal artery. The time lag between the ablation and blood sampling was constant in all patients. We collected a total of eight blood samples from each patient. The probes were stored directly on ice in the catheterisation laboratory and processed within 10 minutes with immediate centrifugation and freezing. Epinephrine and NE plasma levels were assessed with high performance liquid chromatography (HPLC) using the Waters Alliance HPLC 2695 system and the M460 detector (Waters GmbH, Eschborn, Germany) as described previously11,12. Samples were assessed in batches, and all samples from a specific patient were assessed together.
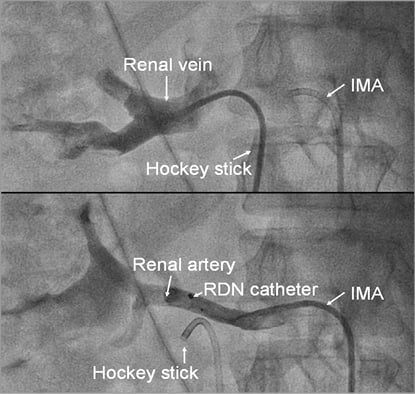
Figure 1. Angiogram of the renal artery and vein for selective blood sampling. Angiogram of the renal vein, performed with a hockey stick guiding catheter, and of the renal artery, performed with an internal mammary guiding catheter (IMA). Selective blood samples were drawn directly after the respective angiograms, ensuring correct catheter position.
Pre- and post-procedural NE and epinephrine (E) concentrations were measured from samples obtained selectively of each renal artery and vein. The veno-arterial difference was defined as NE gradient and E gradient, respectively. The NE outflow in the renal vein (NEvein) equals the sum of the inflow in the renal artery (NEartery) plus the NE from renal nerves (NEnerve) within the kidney minus the NE metabolisation (uptake and degradation, NEM). Assuming a constant blood flow and energy-dependent NE metabolism, the main changes during RDN are related to NEnerve due to renal ablation. By subtracting the pre-procedural from the post-procedural values, the resulting equation yields: change of veno-arterial NE gradient=(Post NEnerve)-(Pre NEnerve)=Post(NEVein - NEartery) - Pre(NEVein - NEartery) (Figure 2).
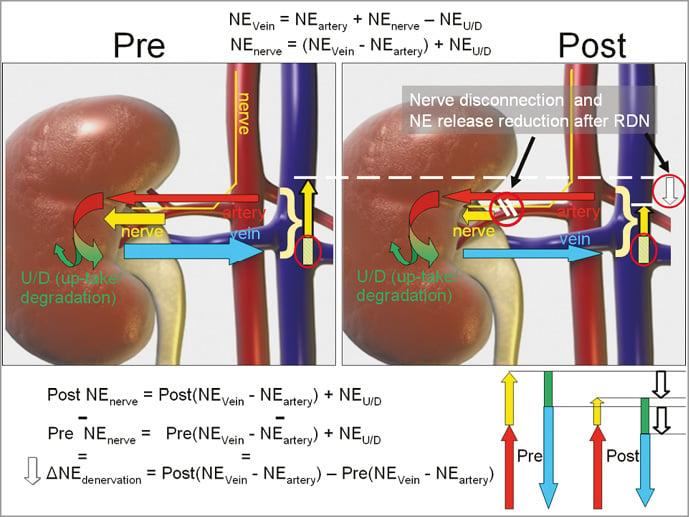
Figure 2. Assessment of the periprocedural reduction (white arrow, ΔNEdenervation) of the veno-arterial NE gradient. The total inflow of catecholamines through the renal artery (red arrow) and through the renal sympathetic nerves (yellow arrow) equals the catecholamine venous outflow (blue arrow) minus the catecholamine metabolisation (uptake and degradation, green arrow/bar) in the figure and schematic diagram for the pre- and post-RDN status.
In order to evaluate possible differences in the renal blood flow, two independent operators assessed all renal artery angiograms. Renal blood flow velocity was assessed as previously published13. In detail, the number of cine frames was counted before and after the RDN procedure from the first frame with contrast dye entering the renal artery until the dye reached the renal cortex. The heart rate was documented before and directly after RDN during blood sampling.
STATISTICAL ANALYSIS
Office blood pressure (OBP) responders were defined as patients with a ≥10 mmHg reduction of the office systolic blood pressure (SBP). ABP responders were defined as patients with ≥10 mmHg reduction of the mean daytime systolic ABP. Sample size calculation was based on the following assumptions: we assumed an office SBP decrease of 15 mmHg at follow-up and a BP responder rate (≥10 mmHg SBP decrease) of 50% based on the available catecholamine data from the SYMPLICITY HTN-1 trial. We further assumed a reduction of the NE gradient of 30% on average, 45% in the responder group and 20% in the non-responder group. Accordingly, using a two-sided 5% significance level and a 90% power, a total of 44 patients was needed. To account for possible losses to follow-up and incomplete catecholamine measurements, a total of 50 patients was scheduled for enrolment. We assessed baseline and procedural characteristics and medication compliance regarding a putative impact on the BP response. Categorical variables such as demographics and medical history data were summarised using frequencies and proportions and were compared using the chi-square test or Fisher’s exact test, as appropriate. Each distribution of values was tested for normality using the Shapiro-Wilk test. The H0 hypothesis of normality was rejected for p-values <0.05. Continuous data were summarised using mean±standard deviation and compared using the Student’s t-test for normally distributed values. Values without a normal distribution were presented using the median (25th, 75th percentiles) and compared using the nonparametric Wilcoxon rank-sum test. Periprocedural catecholamine changes and pre-procedural to three-month follow-up blood pressure changes per patient were compared using the paired t-test, and NE and BP responders using the paired sample sign test. P-values <0.05 were considered statistically significant. Analysis was carried out with SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
BLOOD PRESSURE REDUCTION
Complete bilateral norepinephrine (NE) and epinephrine (E) measurements and complete BP and ABP assessments were available for 46 patients out of the 50 included patients (92%). Despite careful handling of the blood samples, the probes from four patients harvested selectively from the renal artery and veins during the procedure did not reach the central laboratory. Therefore, the NE and E values were not available for these four patients. They could not be included in the catecholamine evaluation despite their informed consent. These four patients had a similar reduction of their systolic office BP of 10 mmHg at three months. The 46 patients with complete catecholamine data comprised the study population. Office systolic BP decreased from 176±19 mmHg to 165±24 mmHg at three months (p=0.02). The mean systolic ABP decreased by 5 mmHg (p=0.03) and the maximum systolic ABP by 9 mmHg at three months (p=0.02). Twenty-six patients out of 46 (57%) were OBP responders with ≥10 mmHg reduction of the office SBP at three months. There was no change in diastolic BP parameters. There was no change in the pulse rate, either when comparing the mean, maximum or minimal heart rate, or by day or at night. No serious adverse event occurred in our study population. The daytime mean systolic ABP was reduced by 7 mmHg compared to baseline (p=0.03).
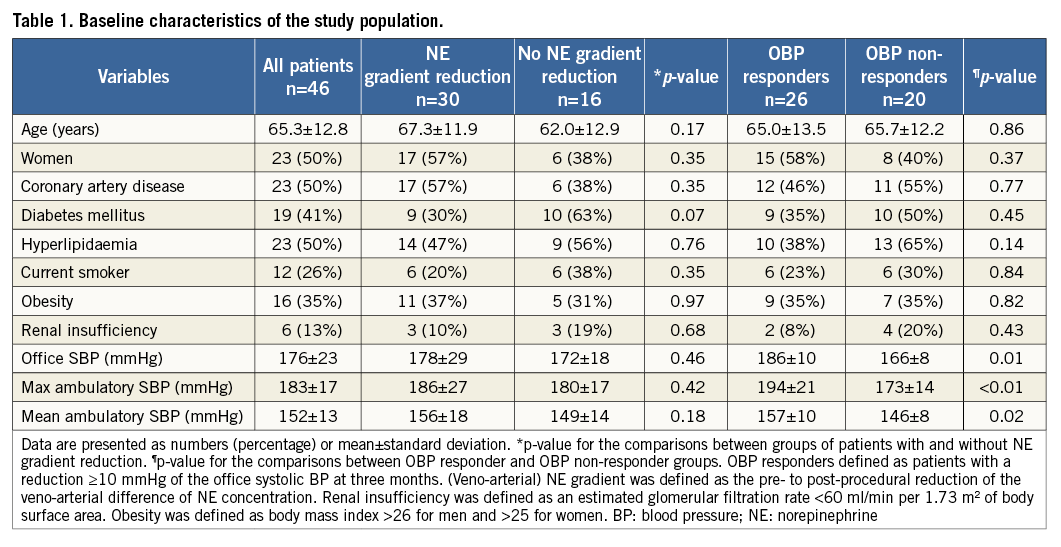
Age, gender and distribution of cardiovascular risk factors were similar when comparing patients with or without reduction of the veno-arterial NE gradient or OBP responders and non-responders, respectively (Table 1). Baseline office SBP, and mean and maximum systolic ABP measurements were higher in OBP responders compared to non-responders based on the allocation of patients to the respective groups. However, these baseline BP measurements were not different comparing patients with or without reduction of the veno-arterial NE gradient, respectively.
The office systolic blood pressure at six months, available for 40 patients (87%), was 163±22 mmHg, representing a reduction of 13 mmHg compared to baseline (p=0.02). The responder rate was 65% (26 out of 40 patients). The mean systolic ABP at six months, assessed for 37 patients, decreased by 6 mmHg compared to baseline (p=0.03).
There were no significant BP changes at follow-up compared to baseline in patients undergoing atrial ablation (p>0.09).
VENO-ARTERIAL NE GRADIENT AND ASSOCIATION WITH BP RESPONSE
The inter-assay coefficients of variation were 4.8% for epinephrine, 2.6% for norepinephrine, 3.9% for metanephrine and 7.3% for normetanephrine. The limit of quantification was 15 pg/ml for epinephrine/norepinephrine, 4.65 pg/ml for metanephrine and 4.13 pg/ml for normetanephrine. The linearity was 10,000 pg/ml for epinephrine/norepinephrine and 5,000 pg/ml for metanephrine/normetanephrine. Higher values above were displayed as >10,000 pg/ml and >5,000 pg/ml, respectively.
We documented a significant decrease in norepinephrine RV-RA difference from pre- to post-procedural levels (median 186 pg/ml [54;466] vs. 81 pg/ml [0;182], p=0.02) (Figure 3). In contrast, there was no significant change of the epinephrine concentrations (median 0 pg/ml [–20;24] vs. 0 pg/ml [–13;32], p=0.31).
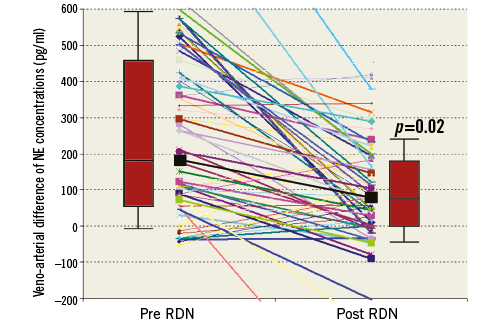
Figure 3. Periprocedural veno-arterial NE difference. Individual veno-arterial difference of NE concentrations during RDN. Thick lines represent values for OBP responders. The thick black line and the box plots display the median (interquartile range) of the veno-arterial NE with a significant reduction during RDN (p=0.02).
The main result of our study is the significant association of the periprocedural veno-arterial NE gradient reduction with the office SBP decrease at three months. OBP responders showed a markedly higher NE gradient reduction (–290±450 pg/ml vs. –4±106 pg/ml, p=0.01) (Figure 4). The Pearson correlation coefficient is 0.5 for this association (p=0.09). The association with the NE gradient reduction was also significant when the definition of the responder status (≥10 mmHg reduction) was based on the mean daytime systolic ABP at three months (-338±493 pg/ml vs. –27±188 pg/ml, p=0.02) (Figure 5) and at six months (–275±472 pg/ml vs. –37±218 pg/ml, p=0.04). This association remained significant for the systolic OBP (≥10 mmHg reduction) at six months (–268±387 pg/ml vs. –44±232 pg/ml, p=0.03) (Figure 6).
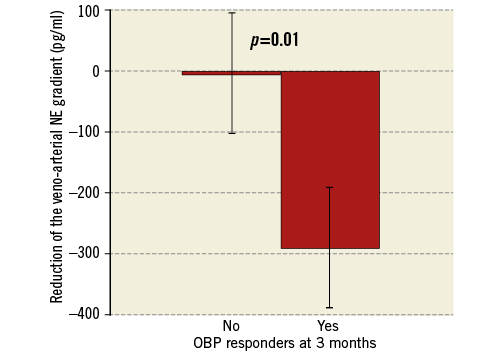
Figure 4. Higher reduction of the veno-arterial NE gradient in OBP responders. OBP responders were defined as patients with a reduction ≥10 mmHg of the office systolic BP at three months. Veno-arterial NE gradient was defined as the pre- to post-procedural reduction of the veno-arterial difference of NE concentration. Bars show standard error of the mean. NE: norepinephrine; OBP: office systolic blood pressure
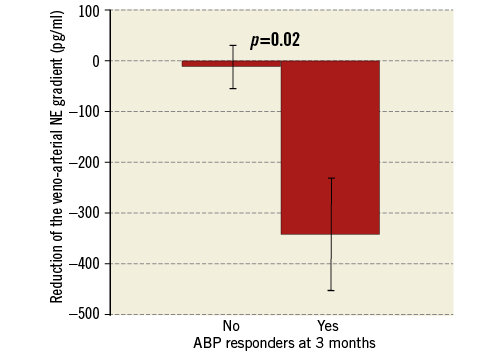
Figure 5. Higher reduction of the veno-arterial NE gradient in ABP responders. ABP responders were defined as patients with a reduction ≥10 mmHg of the mean daytime systolic ABP at three months. Veno-arterial NE gradient was defined as the pre- to post-procedural reduction of the veno-arterial difference of NE concentration. Bars show standard error of the mean. ABP: ambulatory blood pressure; NE: norepinephrine
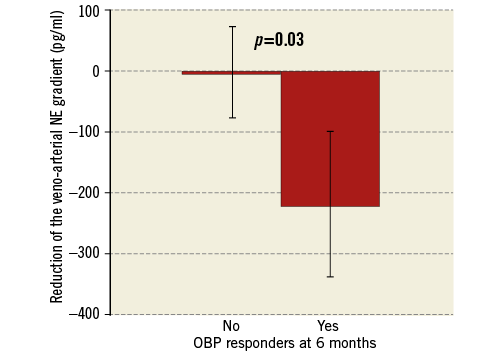
Figure 6. Higher reduction of the veno-arterial NE gradient in OBP responders at 6 months. NE: norepinephrine; OBP: office systolic blood pressure
Patients with a reduction in the NE gradient in both kidneys exhibited the highest SBP reduction (–22±14 mmHg, p=0.03), while patients with a reduction in only one kidney (–5±15 mmHg) or patients without NE gradient reduction (–3±19 mmHg) displayed no significant change in office SBP (Figure 7). Similar results were found when overall and daytime ABP measurements were analysed. This suggests the importance of achieving adequate sympathetic denervation in both kidneys.
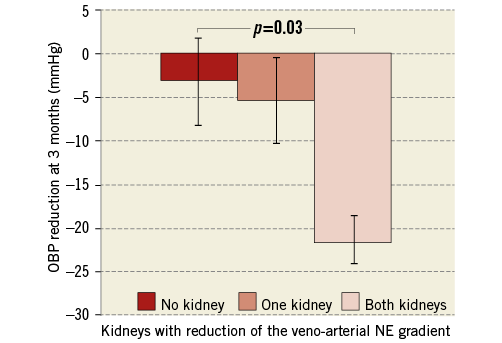
Figure 7. Number of kidneys with veno-arterial NE gradient reduction and corresponding BP response. The extent of the OBP response at three months was significantly associated with the number of kidneys with NE gradient reduction. Veno-arterial NE gradient was defined as the pre- to post-procedural reduction of the veno-arterial difference of NE concentration. OBP responders were defined as patients with a reduction ≥10 mmHg of the office systolic BP at six months. Bars show standard error of the mean. NE: norepinephrine; OBP: office systolic blood pressure
For the group of 20 patients undergoing ablation for atrial fibrillation with a similar regimen of analgosedation, there was no difference comparing pre- and post-procedural arterial levels of NE (pre: 306±425 pg/ml vs. post: 331±614 pg/ml, p=0.32).
IMPACT OF ADDITIONAL PROCEDURAL CHARACTERISTICS
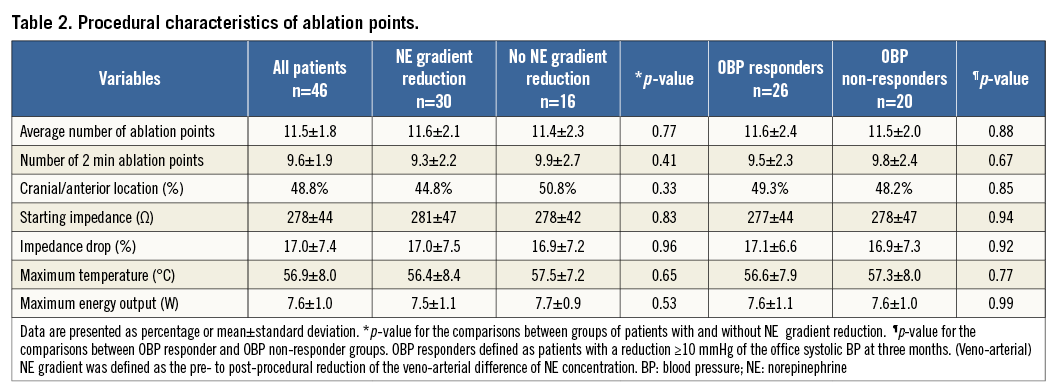
The sequence of treated kidneys, the number of ablation points, their location, energy delivered, overall and single point duration, temperature rise, or impedance drop were all not significantly different comparing patients with or without NE gradient reduction or OBP responders with OBP non-responders in the univariate testing (Table 2).
Considering a potential impact of analgosedation on the sympathetic nerve activity, we analysed the heart rate and found no difference before and after RDN (66±10 vs. 66±8 beats per minute [bpm], p=0.97). There was no difference between the heart rate before and after RDN in patients with NE gradient reduction (64±10 vs. 64±8 bpm, p=0.65), in patients without NE gradient reduction (69±10 vs. 69±9 bpm, p=0.71), in OBP responders (64±11 vs. 65±8 bpm, p=0.73) and in BP non-responders (64±10 vs. 64±11 bpm, p=0.99).
Differences of renal blood flow before and after RDN could alter plasma catecholamine concentration. However, there was no significant difference between the arterial blood flow reflected by the renal artery frame count before and after the procedure for the entire population (8.9±3.5 vs. 8.7±3.3, p=0.33). Similarly, renal artery frame count before and after RDN was not different for patients with NE gradient reduction (9.2±3.5 vs. 8.8±3.4, p=0.14), for patients without NE gradient reduction (8.5±3.5 vs. 8.7±3.6, p=0.56), for OBP responders (9.0±3.4 vs. 8.9±3.6, p=0.92) and for BP non-responders (8.8±3.8 vs. 9.0±3.6, p=0.86). The mean length of the renal artery before reaching the cortex was 97±26 mm. The mean lumen diameter of the renal artery after nitroglycerine application did not differ before and after RDN (pre: 6.2±1.6 mm vs. post: 6.2±1.7 mm, p=0.93). After correction for the calculated blood flow, the veno-arterial NE gradient was still significantly associated with the BP response (p=0.04). We limited the amount of contrast dye to 52±13 ml per patient, without difference between patients with or without NE gradient reduction or between OBP responders vs. non-responders (p>0.11).
ANTIHYPERTENSIVE MEDICATION AND LABORATORY RESULTS
Patients were taking an average of 5.2±1.6 antihypertensive medications before RDN and 5.0±1.4 at three months after RDN (p=0.69) (Table 3). There were no significant differences when comparing the different antihypertensive medications at baseline and at follow-up for the overall population and for the specific subgroups (Table 3).
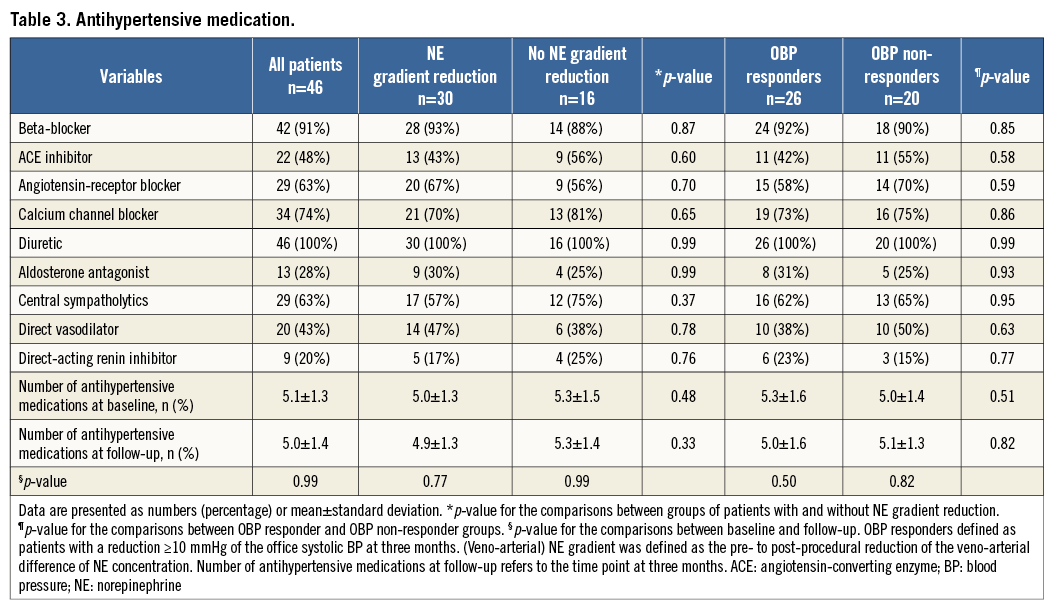
A number of potential markers of RDN success were measured with no substantial differences between OBP responders and OBP non-responders (Table 4).
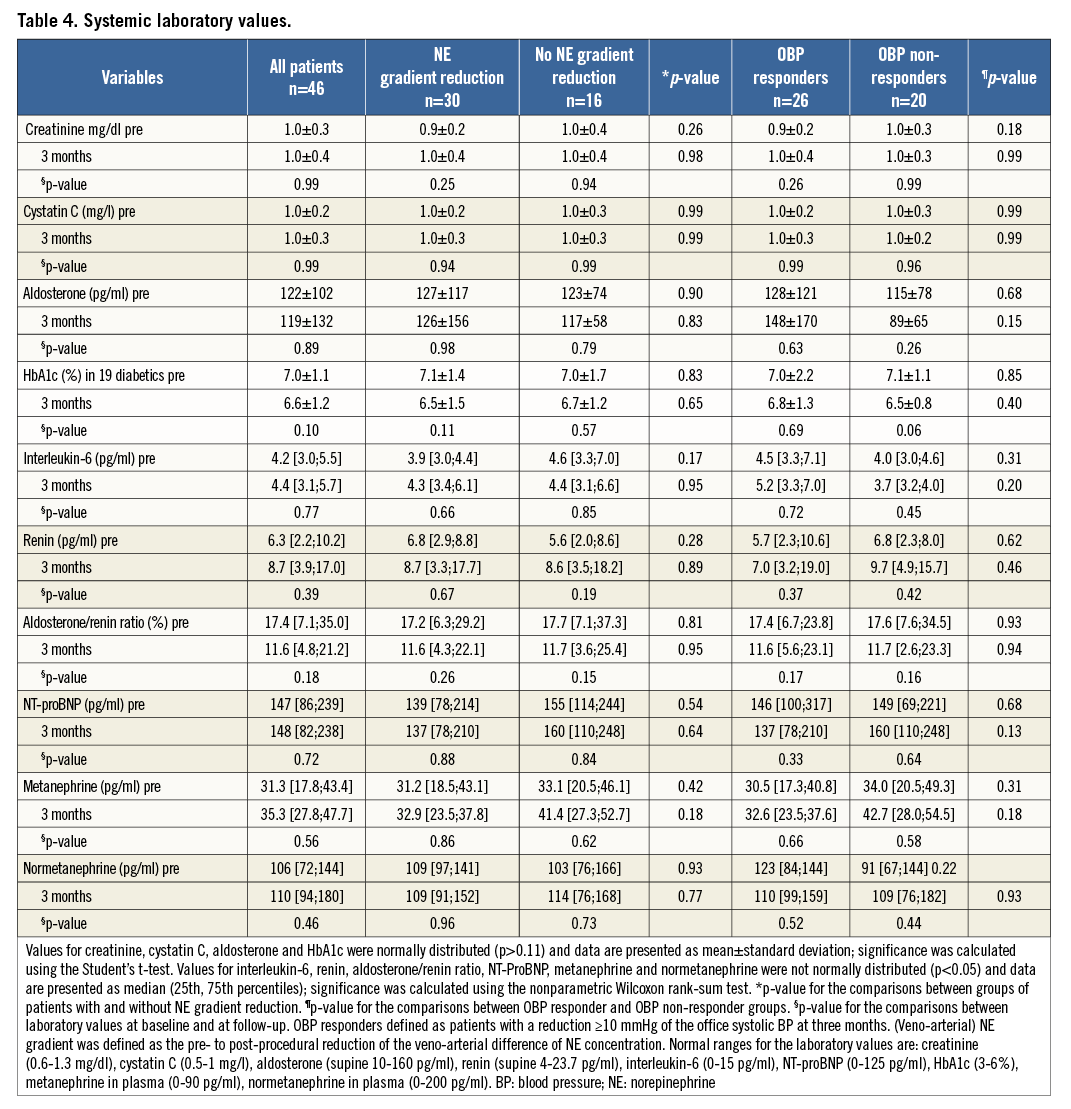
As specified by the study protocol, we determined the pre- and post-procedural catecholamines in the selective arterial and venous samples and calculated the veno-arterial NE gradient before and after RDN (Table 5). The veno-arterial NE gradient was significantly associated with the decrease of the systolic BP, the main finding in our study.
In the multivariate analysis with baseline office BP, the veno-arterial NE gradient reduction, body mass index, and medication status in the model, the significant predictors of the office SBP reduction were baseline office BP, with an odds ratio of 0.43 (0.07 to 0.82; p=0.02), and the veno-arterial NE gradient reduction, with an odds ratio of 0.52 (0.09 to 0.95; p=0.04).
Discussion
To our knowledge, this study showed for the first time a significant impact of the periprocedural renal veno-arterial NE gradient reduction during RDN on the systolic OBP and systolic ABP response at three and six months. In addition, a significant systolic BP reduction was observed only in patients with a decrease in veno-arterial NE gradient in both kidneys. No such differences were observed in patients undergoing ablation for atrial fibrillation under similar sedation with propofol and fentanyl.
COMPARISON WITH PREVIOUS STUDIES
The landmark SYMPLICITY HTN-3 trial found a significant BP reduction of approximately 14 mmHg for the office SBP and 7 mmHg for the mean systolic ABP after six months in the RDN group without a significant difference to the sham group7. Possible explanations include an incomplete denervation given the heterogeneous distribution of sympathetic nerves and the inter-individual variability8.
However, the optimal modulation of the sympathetic nerve system is still poorly understood and challenging to assess. Previous data suggest that NE in plasma is derived largely from transmitter release by sympathetic nerves, with the heart and the kidney having the highest changes of the veno-arterial NE gradient10.
A subgroup analysis of 10 patients from the SYMPLICITY HTN-1 trial showed a 47% reduction of the NE spillover assessed by the isotope dilution method two weeks after RDN2. These results suggested an inhibition of the renal sympathetic system at two to four weeks after RDN, but did not allow any correlation between the immediate procedural NE changes and the BP response1,14,15.
The reductions of the office SBP of 11 mmHg and 12 mmHg and mean systolic ABP of 5 mmHg and 6 mmHg at three months and six months are comparable to the results in the SYMPLICITY HTN-3 trial7. Our OBP responder rate, defined as patients with an office SBP reduction ≥10 mmHg, was 57% at three months and 65% at six months, comparable to results from recently published trials, but less than in the SYMPLICITY HTN-1 and HTN-2 studies1-3,7,16,17.
OFFICE BP VS. ABP MEASUREMENTS AND ASSOCIATION WITH THE VENO-ARTERIAL NE GRADIENT
In addition to our primary endpoint based on the office systolic BP, we evaluated a possible association of changes of the veno-arterial NE gradient with ABP measurements1,3,6. The ABP approach reduces observer bias, improves reproducibility, and minimises the white coat effect4-7,18,19. Similarly to the office SBP, we found a highly significant association of the reduction of systolic ABPMs with changes of the veno-arterial NE gradient. The association of the veno-arterial NE gradient with OBP and ABP responder status at three and six-month follow-up strengthens the findings of our study.
EVALUATION OF LABORATORY PARAMETERS AND ANTIHYPERTENSIVE MEDICATION
Different effects of the RDN procedure on renal function or glycaemic control were postulated in previous studies4,20. In our study, however, we did not find any relevant differences (Table 4).
Importantly, there was no difference in systemic catecholamine concentrations in our study, showing that the peripheral plasma levels are unreliable predictors of the efficacy of the RDN procedure. These results are contradictory to a recently published study with a total of five patients with significant changes of these parameters depending on the blood pressure responder status21. Similarly to our study, the investigators found no differences for creatinine, aldosterone or renin after RDN21.
Limitations
The population of 46 patients and the variability of catecholamine measurements are limitations of our study. Confirmatory studies are needed to validate our findings further. A fast availability of catecholamine measurements would allow an expeditious decision regarding the need for additional ablation.
Another limitation is the lower mean 24-hour SBP of 152±13 mmHg in our study compared to 159±13 mmHg in the SYMPLICITY HTN-3 trial.
The lack of an association of the total number of ablation points with the NE gradient reduction of BP response suggests that not the number, but rather the proximity of the ablation point to the sympathetic nerve strands determines an effective ablation.
Additional limitations are related to the arousal status and blood flow changes during RDN due to contrast dye and the ablation itself, influencing the measured concentrations of catecholamines. However, we found no significant difference between the pre- and post-procedural arterial E concentration, suggesting a constant arousal status. Similarly, the heart rate did not change during RDN, either for the entire population or for specific subgroups. Regarding the renal blood flow, there was no significant change of the venous and veno-arterial E levels from the pre- to post-procedural status, suggesting no relevant changes of the blood flow, and therefore no significant influence on the catecholamine concentrations. In addition, there were no changes of the renal blood flow assessed by the renal arterial frame count for the entire population and the specific subgroups. However, the frame count as a surrogate parameter for flow is validated for coronary arteries, but not for renal arteries.
Finally, no differences in arterial NE levels and blood pressure were observed in patients undergoing ablation for atrial fibrillation.
Conclusions
In summary, our study identified for the first time the periprocedural reduction of the veno-arterial NE gradient, reflecting the direct effect of the renal denervation procedure. This periprocedural veno-arterial NE gradient reduction is associated with the blood pressure decrease at three and six months after RDN. This may be a method to gauge the extent of renal nerve ablation.
| Impact on daily practice The assessment of the renal veno-arterial NE gradient during RDN is feasible and safe, based on selective arterial and venous blood sampling before and after the RDN procedure. The periprocedural reduction of renal veno-arterial NE gradient is associated with the BP response after RDN. The information gained through this assessment may lead to improved outcome after renal denervation. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

