Abstract
Aims: It has been argued that hyperaemic microvascular resistance (HMR), defined as the ratio of mean distal coronary pressure to flow velocity, is overestimated in the presence of a coronary stenosis compared to actual microvascular resistance (MR), due to neglecting collateral flow. We aimed to test the hypothesis that HMR allows accurate identification of microvascular functional abnormalities by evaluating the association between high or low HMR and the presence of myocardial ischaemia on non-invasive stress testing.
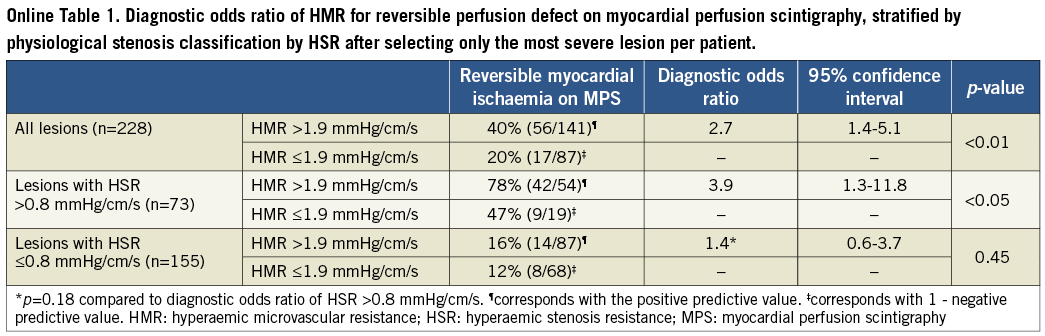
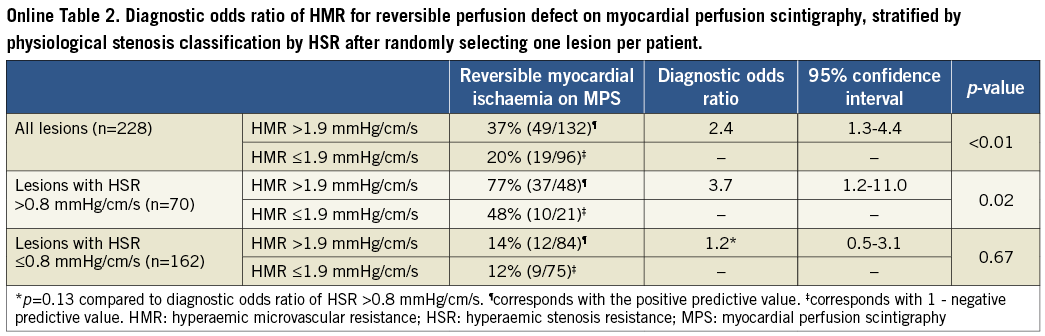
Methods and results: Myocardial perfusion scintigraphy was performed in 228 patients, with 299 lesions to identify reversible myocardial ischaemia. Intracoronary distal pressure and flow velocity were assessed during adenosine-induced hyperaemia (20-40 µg, intracoronary) to determine hyperaemic stenosis resistance (HSR) and HMR. HMR >1.9 mmHg/cm/s was defined as high. The diagnostic odds ratio (OR) for myocardial ischaemia for lesions associated with high compared to low HMR was 2.6 (95% confidence interval [CI]: 1.5-4.4; p<0.001) overall, 3.3 (95% CI: 1.2-9.0; p=0.02) for lesions with HSR >0.8 mmHg/cm/s, and 1.3 (95% CI: 0.6-2.9; p=0.52) for lesions with HSR ≤0.8 mmHg/cm/s.
Conclusions: The increased risk of myocardial ischaemia in the presence of high HMR, uncorrected for collateral flow, demonstrates that HMR is reflective of an increase in actual MR, identifying pertinent pathophysiological alterations in the microvasculature.
Introduction
Coronary microvascular disease is considered an essential component in the spectrum of ischaemic heart disease, and is likely associated with altered mechanical and functional properties of the microcirculation, affecting minimal resistance of the coronary microvasculature1. Since there is no technique currently available which allows direct visualisation of the coronary microcirculation in vivo, the hyperaemic microvascular resistance index (HMR), defined as the ratio of hyperaemic mean distal coronary pressure to mean distal coronary flow velocity, has been proposed as a surrogate means to quantify the functional status of the microvasculature. However, on theoretical grounds it has been argued that HMR overestimates the magnitude of actual microvascular resistance (MR) in the presence of a flow-limiting coronary stenosis due to neglecting the collateral flow contribution to total myocardial blood flow2,3 (Figure 1). After correcting for collateral flow, microvascular resistance would then be minimal and constant2-5, i.e., HMR would be equal to actual MR.
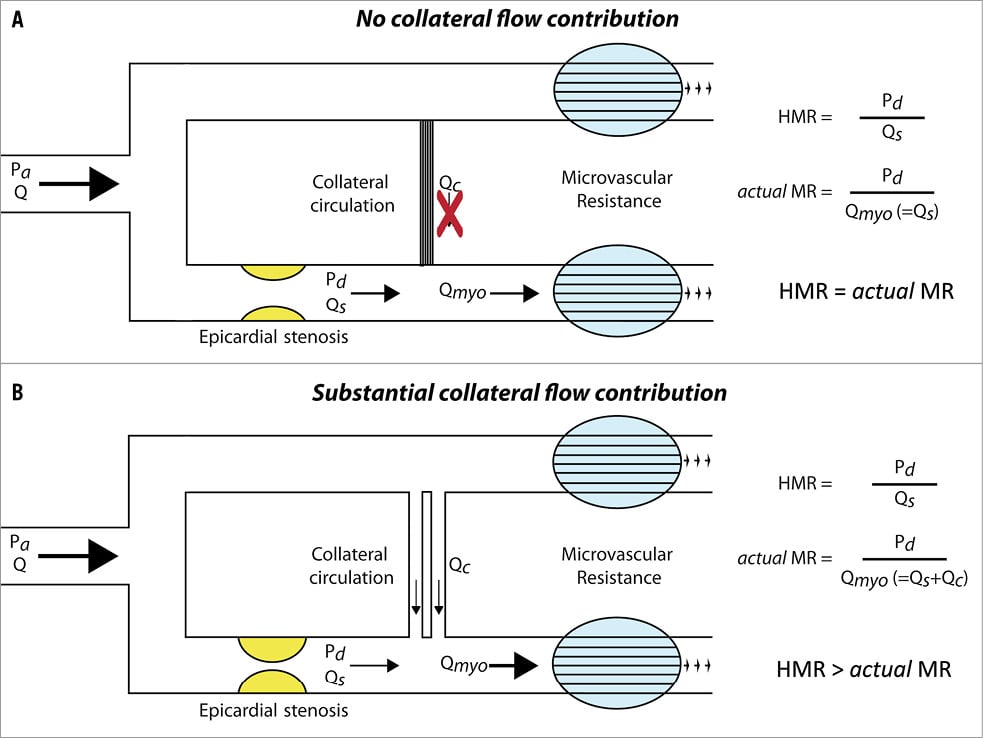
Figure 1. Schematic representation of the effect of neglecting collateral flow on the calculation of the microvascular resistance index. In the absence of collateral flow (Qc), distal coronary flow (Qs) equals total myocardial blood flow (Qmyo), and the hyperaemic microvascular resistance (HMR) index equals actual microvascular resistance (MR) (A). In the presence of collateral flow, Qs underestimates Qmyo due to neglecting the contribution of Qc, and HMR overestimates actual MR (B). Pa: aortic pressure; Pd: distal coronary pressure; Q: coronary flow
Pathophysiological alterations in the coronary microcirculation contribute to the occurrence of myocardial ischaemia1,6,7. In case microvascular resistance in the presence of an epicardial narrowing would indeed be minimal and constant after correcting for collateral flow contribution, and hence an increased HMR would only reflect an overestimation of its true magnitude, one would not expect a positive relationship between such an increase in HMR and the presence of reversible myocardial ischaemia. We aimed to determine whether HMR, according to its original definition, allows accurate identification of pathophysiological alterations in the coronary microcirculation, obviating the need for correcting HMR for collateral flow contribution. We therefore evaluated the association between high or low HMR and the risk of reversible myocardial ischaemia on non-invasive stress testing in the absence and presence of flow-limiting coronary stenoses.
Methods
DATA SOURCE
Between April 1997 and September 2006, we evaluated patients referred for intracoronary evaluation of at least one coronary stenosis of intermediate severity (40-70% diameter stenosis on visual assessment). These patients were enrolled in a series of study protocols8-11, and patient and procedural characteristics were entered into a dedicated database. We excluded patients with ostial lesions, ≥2 stenoses in the same coronary artery, severe renal function impairment (sMDRD calculated glomerular filtration rate <30 mL/min/1.73 m2), significant left main coronary artery stenosis, atrial fibrillation, recent myocardial infarction (<6 weeks before screening), or prior coronary artery bypass graft surgery. The institutional ethics committee approved the study procedures, and all patients gave written informed consent.
MYOCARDIAL PERFUSION SCINTIGRAPHY
MPS was performed to document the presence of reversible perfusion defects, using either 99mTechnetium-sestamibi (MIBI) or 99mTechnetium-tetrofosmin (Myoview; GE Healthcare, Little Chalfont, Buckinghamshire, UK), according to a two-day stress-rest protocol. Stress was induced either pharmacologically by adenosine or dipyridamole, or by exercise. Data acquisition and reconstruction were performed according to the procedure guideline for myocardial perfusion imaging of the Society of Nuclear Medicine12. An expert panel of nuclear medicine physicians, blinded to the angiographic data, evaluated the scintigraphic images. Perfusion defects were classified as dubious, mild, moderate or severe. Improvement at rest of more than one grade was considered to be a “reversible” perfusion defect. Improvement of just one grade or no improvement was considered to be a “persistent” perfusion defect. The result was considered positive when a reversible defect was allocated to the perfusion territory of the coronary artery of interest.
CARDIAC CATHETERISATION
All patients underwent cardiac catheterisation within one week after MPS. Coronary angiography was performed according to standard procedures, and angiographic images were obtained in a manner suitable for quantitative coronary angiography (QCA) analysis. Distal coronary pressure and blood flow velocity were consecutively assessed with separate sensor-equipped guidewires (Volcano Corp., San Diego, CA, USA) during baseline conditions and hyperaemia, induced by an intracoronary bolus of adenosine (20-40 μg). Subsequently, coronary blood flow velocity measurements were performed in a reference coronary artery, defined as a coronary artery with <30% diameter stenosis on visual assessment, when present.
DATA ANALYSIS
In order to determine percent diameter stenosis, offline QCA analysis was performed with the use of a validated automated contour detection algorithm (QCA-CMS version 3.32; Medis, Leiden, The Netherlands). From the haemodynamic data recorded, we determined the hyperaemic stenosis resistance index (HSR), defined as the ratio of the pressure drop across the stenosis to mean distal coronary flow velocity during hyperaemia, fractional flow reserve (FFR), defined as the ratio of distal coronary pressure to mean aortic pressure during hyperaemia, as well as HMR, defined as the ratio of mean distal coronary pressure to mean distal coronary flow velocity during hyperaemia. In the absence of a flow-limiting stenosis within the reference vessels, reference vessel HMR was defined as the ratio of mean aortic pressure to mean distal coronary flow velocity during hyperaemia.
STATISTICAL ANALYSIS
In the absence of a clinically adopted cut-off value or an unequivocal normal range for HMR, the median HMR within the reference vessels was used to distinguish high versus low HMR in the target vessels. At this cut-off value, we evaluated the association between high or low HMR distal to the intermediate stenosis and the presence of reversible perfusion defects on MPS by means of 2x2 contingency tables. Differences were assessed by means of the chi-square test.
Additionally, we evaluated the association between HMR in the target vessel and reversible perfusion defects on MPS after stratification by HSR, applying the ischaemic threshold of >0.80 mmHg/cm/s13. Logistic regression analysis was performed to test for the presence of effect modification of an HSR-positive stenosis on the association between HMR and reversible perfusion defects on MPS. Since a substantial contribution of collateral flow to total myocardial flow is highly unlikely in lesions with an FFR >0.614, the association between HMR distal to coronary stenoses and the presence of reversible perfusion defects on MPS was additionally tested in this subset of lesions. Finally, the association between HMR distal to coronary stenoses and the presence of reversible perfusion defects on MPS was tested at patient level, first by including only the most severe lesion (highest HSR), and secondly by randomly including only one of the lesions, in patients with multivessel disease.
Continuous variables were expressed as mean±standard deviation (SD), or median (25th-75th percentile), and were compared by means of the Student’s t-test or Mann-Whitney U test, where appropriate. Categorical variables were presented as frequency (percentage), and compared with the chi-square test or Fisher’s exact test, where appropriate. A two-sided alpha level of 0.05 was considered statistically significant.
Results
PATIENT POPULATION
The study population consisted of 228 patients, with a mean age of 60±11 years. The baseline clinical characteristics are shown in Table 1. Distal coronary pressure and flow velocity were determined distal to 299 lesions (mean diameter stenosis 55±11%). Within the target vessels, median HMR amounted to 2.1 (1.6-2.8) mmHg/cm/s. A reference vessel was available in 178 patients. The median HMR in the reference vessels was 1.9 (1.5-2.5) mmHg/cm/s, which was subsequently used to stratify HMR.
ASSOCIATION BETWEEN HMR AND MYOCARDIAL ISCHAEMIA ACROSS ALL LESIONS
Overall, lesions associated with a high HMR had a slightly higher percent diameter stenosis on QCA (56±11% when HMR was high versus 53±9% when HMR was low, p=0.03) (Table 2). Nonetheless, epicardial disease severity as identified by FFR did not differ between groups (0.81 [0.70-0.89] when HMR was high versus 0.79 [0.69-0.88] when HMR was low, p=0.46) (Table 2). Despite the fact that FFR did not differ, lesions associated with a high HMR more frequently demonstrated reversible myocardial ischaemia on MPS (37% when HMR was high versus 19% when HMR was low) (Table 3, Figure 2). Accordingly, the diagnostic odds ratio of high versus low HMR for the presence of reversible perfusion defects on MPS was 2.6 (95% confidence interval [CI]: 1.5-4.4; p<0.001) (Table 3). Similar results were found when analyses were performed at patient level (Online Table 1, Online Table 2).
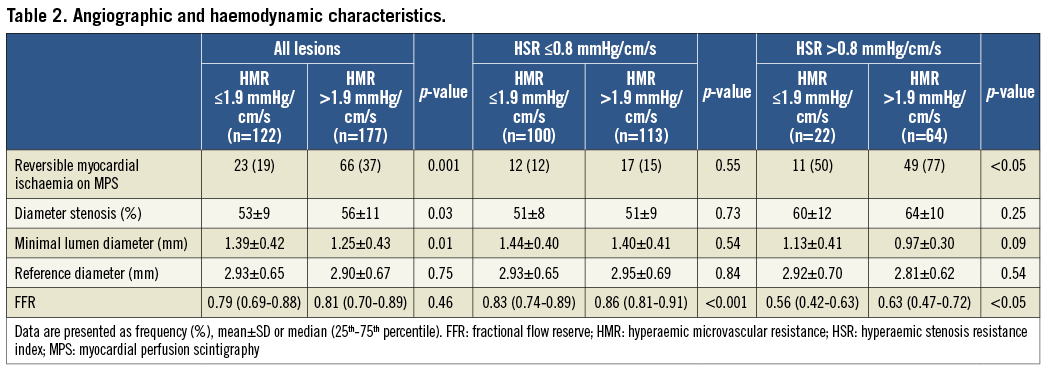
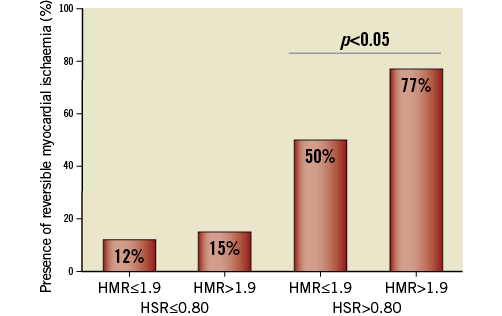
Figure 2. Presence of reversible myocardial ischaemia according to high or low HMR, stratified by HSR. Reversible myocardial ischaemia was most present in functionally significant lesions (hyperaemic stenosis resistance [HSR] >0.8 mmHg/cm/s) and was significantly more present in lesions with a high hyperaemic microvascular resistance (HMR) (77%), compared with lesions with a low HMR (50%) (p<0.05).
ASSOCIATION BETWEEN HMR AND MYOCARDIAL ISCHAEMIA ACCORDING TO HSR
Within physiologically significant coronary lesions (HSR >0.80 mmHg/cm/s), FFR was significantly higher for lesions associated with a high HMR compared with lesions associated with a low HMR (0.63 [0.47-0.72] versus 0.56 [0.42-0.63], respectively; p<0.05) in the absence of differences in percent diameter stenosis (64±10% when HMR was high versus 60±12% when HMR was low, p=0.25) (Table 2). Despite a significantly higher FFR, lesions with a high HMR more frequently demonstrated reversible myocardial ischaemia on MPS compared with lesions associated with a low HMR (77% versus 50%, respectively; p<0.05) (Table 3, Figure 2).
Within lesions with HSR ≤0.80 mmHg/cm/s, FFR was also significantly higher in stenoses associated with a high HMR compared with lesions associated with a low HMR (0.86 [0.81-0.91] versus 0.83 [0.74-0.89], respectively; p<0.001) (Table 2). Nonetheless, no difference in the presence of reversible myocardial ischaemia on MPS was found between lesions associated with a high or low HMR (15% versus 12%, respectively; p=0.55) (Table 3, Figure 2). Accordingly, the diagnostic odds ratio of high versus low HMR for the presence of reversible perfusion defects was 3.3 (95% CI: 1.2-9.0; p=0.02) when HSR was >0.8 mmHg/cm/s, and 1.3 (95% CI: 0.6-2.9; p=0.52) when HSR was ≤0.8 mmHg/cm/s (p for interaction=0.16) (Table 3).
Also after stratifying the data according to HSR, similar results were found when analyses were performed at patient level (Online Table 1, Online Table 2).
ASSOCIATION BETWEEN HMR AND MYOCARDIAL ISCHAEMIA IN LESIONS WITH FFR >0.6
A total of 254 out of 299 coronary stenoses fell within the FFR >0.6 range. Within this FFR range, FFR did not differ between lesions associated with a high or low HMR (0.83 [0.77-0.90], versus 0.81 [0.73-0.88], respectively; p=0.11). When restricting the analysis to stenoses with FFR >0.6, the diagnostic odds ratio of high versus low HMR for the presence of reversible perfusion defects on MPS was 2.9 (95% CI: 1.5-5.8; p<0.01).
Discussion
We observed that a high HMR distal to a coronary artery stenosis was associated with an increased risk for reversible perfusion defects on MPS (diagnostic odds ratio 2.6, 95% CI: 1.5-4.4; p<0.001), which was most pronounced distal to haemodynamically significant coronary stenoses, as identified by HSR. Similar results were found when analyses were performed at patient level. When restricted to stenoses with FFR >0.6, where substantial contribution of collateral flow is highly unlikely14, HMR was similarly associated with an increased risk for reversible perfusion defects on MPS compared to when all lesions were included. This increment in reversible myocardial ischaemia on MPS associated with high HMR was present despite similar, or even less pronounced FFR-identified epicardial disease severity of lesions associated with a high HMR.
These observations imply that HMR, according to its original definition, allows identification of pertinent pathophysiological alterations in the distal microvasculature, and therefore positively confirm that an increase in HMR distal to a coronary stenosis is reflective of an increase in actual MR6,7.
ALTERATIONS IN MICROVASCULAR RESISTANCE RELATED TO CLINICAL PATHOPHYSIOLOGY
The finding in the present study, that a high HMR is associated with an increased risk of related pathophysiology, is consistent with earlier studies using a physiological index of microvascular resistance to document the functional status of the coronary microvasculature. Using either Doppler flow velocity-derived or thermodilution-derived indices of microvascular resistance, an increase in microvascular resistance without correction for potential collateral flow contribution was shown to identify microvascular disease accurately in diabetic patients15, microvascular dysfunction after coronary stenting16, the effect of pharmacological intervention17-19, and microvascular dysfunction in stress-induced cardiomyopathy20. Moreover, it was shown to have important prognostic value in the setting of acute coronary syndrome21-24.
The present study is the first to document that measurement of HMR distal to coronary artery stenoses of intermediate severity allows identification of pertinent pathophysiological alterations in the coronary microvasculature. Our observations thereby support previous conclusions that an increase in minimal microvascular resistance distal to coronary stenoses, as identified by HMR, reflects pertinent alterations in the functional or mechanical properties of the coronary microvasculature6,7.
MECHANISMS OF INCREASED HYPERAEMIC MICROVASCULAR RESISTANCE DISTAL TO A CORONARY STENOSIS
Although we observed that an increased minimal microvascular resistance distal to a coronary stenosis, as assessed by HMR, reflects pertinent alterations in the coronary microvasculature, its precise origin is more difficult to elucidate. Both experimental as well as clinical studies have shown that the coronary vasculature is pressure-distensible at maximal vasodilation, e.g., coronary resistance increases with decreasing perfusion pressure, and vice versa. Hence, with progressive epicardial narrowing, thereby reducing perfusion pressure to the distal vasculature, resistance of the distal vascular bed is likely to increase25-27. Consistent with this pressure-distensibility of coronary resistance vessels, a decrease in microvascular resistance distal to coronary stenoses was reported following restoration of perfusion pressure by stepwise relief of an epicardial stenosis16. However, Table 2 clearly demonstrates that functional lesion severity did not determine HMR in the present study, since a reduced functional lesion severity, as identified by FFR, was found for lesions associated with a high HMR compared to lesions associated with a low HMR. An increase in microvascular resistance may alternatively be explained by several mechanisms not directly related to the epicardial stenosis, including diffuse atherosclerotic disease, endothelial dysfunction, or structural changes in the microvasculature itself, e.g., in the setting of left ventricular hypertrophy7,28. Such structural changes in the microvasculature are acknowledged to contribute to, or even comprise, the sole origin of myocardial ischaemia, and are associated with unequivocal adverse clinical outcome7. Our current findings imply that an increased minimal microvascular resistance distal to coronary stenoses, as identified by HMR, whether following from a fall in perfusion pressure due to the presence of a coronary stenosis, or from abnormalities in the functional or mechanical properties of the microvasculature, or a combination of both, unequivocally represents clinically pertinent coronary pathophysiology within the microvasculature, contributing to the occurrence of myocardial ischaemia.
CORRECTION OF HYPERAEMIC MICROVASCULAR RESISTANCE FOR COLLATERAL FLOW CONTRIBUTION
On theoretical grounds, it has been argued that the definition of HMR, i.e., the ratio of hyperaemic mean distal coronary pressure to mean distal coronary flow, is intrinsically confounded by neglecting the collateral flow contribution to actual myocardial blood flow. Obviously, this is only pertinent should collateral flow be present; HMR then likely overestimates actual MR (Figure 1). Hence, it was suggested that HMR should be corrected for the assumed contribution of collateral flow by means of the coronary wedge pressure, purportedly an estimate of collateral flow. However, two important considerations contradict the application of coronary wedge pressure as an accurate correction factor for collateral flow contribution in clinical practice. First, the magnitude of the coronary wedge pressure is also determined by the effects of heart rate, ventricular wall tension and venous pressure, and cannot be considered an exclusive measure of collateral flow29 (Figure 3). Second, collateral flow contribution is considered to be minimal in the clinically pertinent range of coronary stenosis severity, and to play a substantial role only distal to coronary stenoses with an FFR <0.614. Hence, the use of coronary wedge pressure as a direct measure of collateral flow is anticipated to lead to an overestimation of collateral flow contribution29 and a consequent underestimation of actual MR. Importantly, if an increase in HMR distal to an epicardial stenosis only reflected an overestimation of actual MR due to neglecting collateral flow, one would not expect a positive relationship between such an increase in HMR and reversible myocardial ischaemia, since collateral flow compensatory to progressive epicardial coronary narrowing would not result in an increased presence of reversible myocardial ischaemia, but rather in an equivalent or even a reduced presence. In contrast, the present study showed that a high HMR distal to stenoses of intermediate angiographic and physiological severity was associated with a significantly higher risk for reversible myocardial ischaemia compared to lesions with a low HMR.
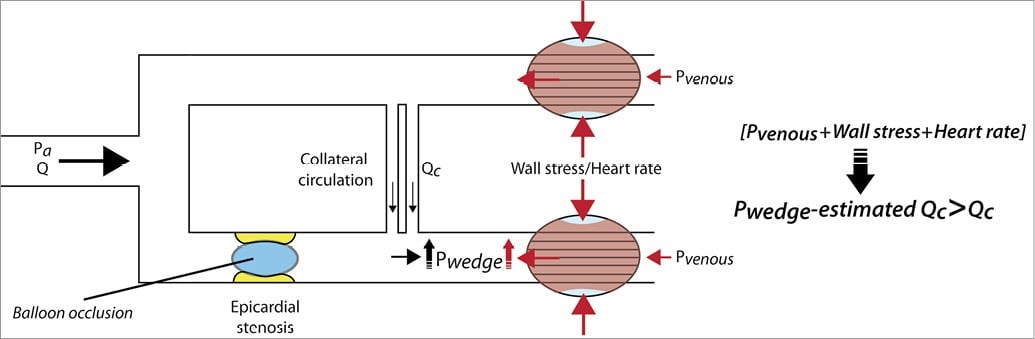
Figure 3. Schematic representation of factors determining coronary wedge pressure. The magnitude of distal coronary pressure obtained during balloon occlusion of the coronary vessel of interest, depicted as coronary wedge pressure (Pwedge), is not only determined by collateral flow (Qc), but is also dependent on venous pressure, wall stress and heart rate. These factors contribute to a higher Pwedge than caused by Qc alone. By using Pwedge as a surrogate for Qc, the actual collateral flow contribution to total myocardial flow is overestimated, resulting in a consequent underestimation of actual MR. The extent of such an underestimation depends on the true magnitude of collateral flow, which cannot be measured directly. Pa: aortic pressure; Q: coronary flow
CLINICAL IMPLICATIONS
Abnormalities in the function and structure of the coronary microcirculation occur in many clinical conditions. They are considered important markers of risk or may contribute to the pathogenesis of myocardial ischaemia, thereby providing novel targets for risk stratification or adjunctive therapeutic strategies28,30. However, in the absence of techniques allowing direct visualisation of the coronary microvasculature in humans in vivo, evaluation of microvascular abnormalities in clinical practice requires an accurate surrogate. Since microvascular disease is likely associated with altered mechanical and functional properties of the microcirculation, thereby affecting resistance of the coronary microvessels, physiological indices of hyperaemic microvascular resistance are increasingly used to quantify the functional status of the coronary microvasculature in the cardiac catheterisation laboratory. As such, the potential error induced either by neglecting collateral flow contribution, or by overestimating its magnitude by applying a coronary wedge pressure-based correction, may hamper the clinical use of an index of microvascular resistance. Because actual MR is unprocurable in vivo, an applicable index most importantly allows accurate assessment of pathophysiological alterations in the distal microvasculature. Consistent with earlier studies15,16,20,22-24,26,31,32, our observations indicate that the microvascular resistance index calculated without correction for assumed collateral flow contribution allows identification of alterations in microvascular resistance that are associated with an increased risk of associated pathology. HMR may therefore be considered a useful tool to quantify the functional status of the coronary microvasculature in clinical practice.
STUDY LIMITATIONS
Whereas developments in wire technology have resulted in the availability of a double sensor-equipped guidewire33, pressure and flow velocity measurements were performed sequentially with separate sensor-equipped guidewires in the present study, which could have an inherent bias due to a possible location shift between the measurements. However, the wire tip location was verified angiographically between measurements to ensure representative pressure and flow velocity signals.
A true gold standard for the assessment of reversible myocardial ischaemia in clinical practice is not available. Therefore, we used MPS to evaluate the presence of reversible myocardial ischaemia in our study population. Despite its associated limitations, especially in the presence of multivessel disease, MPS is considered a well-validated method to document perfusion abnormalities in clinical practice. Importantly, similar results were found when analyses were performed including all lesions as compared to only the most severe lesion, or when randomly selecting only one of the lesions in patients with multivessel disease, indicating that our conclusions were not compromised by the presence of multivessel disease.
There is contrasting evidence on the appropriate dose of adenosine required to induce a maximal hyperaemic state. In the present study, we adhered to the amount used in clinical validation studies of FFR that is considered to provide a hyperaemic response equivalent to that induced by the intravenous administration of adenosine34.
Finally, we only evaluated the relationship between the coronary flow velocity-derived HMR and myocardial ischaemia. Theoretically, the same observations apply to the thermodilution-derived index of microvascular resistance (IMR), since the only difference is the surrogate of flow applied (velocity versus inverse of transit time). Nevertheless, extrapolation of our results to IMR should be carried out with caution, and confirmation of our results is required in studies with a thermodilution-derived surrogate of flow.
Conclusion
A high HMR distal to a coronary stenosis, calculated without correction for assumed collateral flow contribution, is associated with an increased risk of reversible myocardial ischaemia in the perfusion territory of interest. This observation indicates that an increased HMR distal to a coronary stenosis reflects important pathophysiological alterations in the distal microvasculature in the setting of obstructive coronary artery disease. HMR may therefore be a useful tool to quantify the functional status of the myocardial microvasculature in clinical practice.
| Impact on daily practice Currently no technique is available which allows direct visualisation of the coronary microcirculation in vivo. From our observations it can be concluded that an increase in minimal microvascular resistance distal to coronary stenoses, as identified by HMR, reflects pertinent alterations in the functional or mechanical properties of the coronary microvasculature, and can therefore be used as a surrogate means to quantify the functional status of the microvasculature in daily clinical practice. |
Acknowledgements
This work was supported by the Netherlands Heart Foundation (grant numbers 2000.090 and 2006B186) and the European Community’s Seventh Framework Programme (grant number FP7-ICT-2007-224495: euHeart).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement