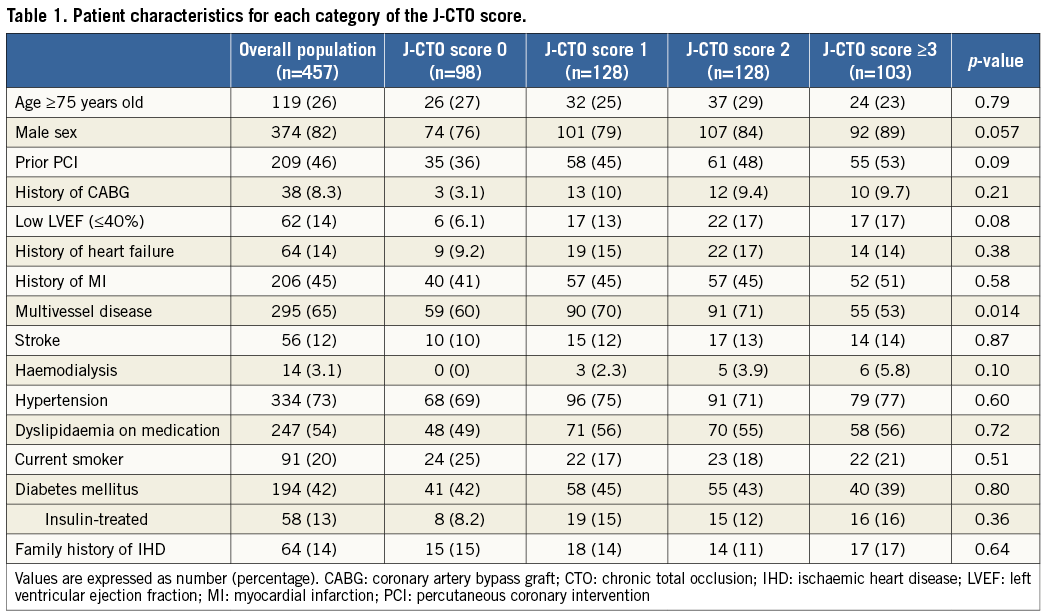
Abstract
Aims: We investigated the impact of the J-CTO score, a pre-procedural risk score for successful guidewire crossing within 30 minutes through chronic total occlusion (CTO) lesions, on procedural and midterm clinical outcomes in terms of target lesion revascularisation (TLR) after CTO recanalisation.
Methods and results: The primary endpoint of this substudy was midterm TLR. The net midterm success rate was calculated by multiplying the lesion success rate by the TLR-free survival rate. The initial lesion success rates according to the J-CTO score categories of 0, 1, 2, and ≥3 were 97.0%, 92.1%, 86.5%, and 73.6%, respectively (p<0.001). The TLR rates at one year according to the J-CTO score categories of 0, 1, 2, and ≥3 were 5.3%, 11.1%, 16.7%, and 13.4%, respectively (p=0.082). The net midterm success rates according to the J-CTO score categories of 0, 1, 2, and ≥3 were 91.9%, 81.9%, 72.1%, and 63.7%, respectively (p<0.001).
Conclusions: Patients with CTO lesions with lower J-CTO scores are expected to achieve a high procedural success rate and an increased TLR-free survival rate. Patients with high J-CTO scores still remain an issue.
Introduction
Successful percutaneous coronary intervention (PCI) for chronic total occlusion (CTO) is associated with improvement in quality of life and left ventricular function as well as a reduced need for coronary artery bypass grafting (CABG)1-7. In addition, observational studies have suggested improvement in the survival rates after successful PCI for CTO compared with unsuccessful PCI1-4,8.
Recently, we reported that the J-CTO score, a scale based on pre-procedural clinical and angiographic factors, could predict the probability of successful guidewire (GW) crossing of CTO lesions9. According to this study, CTO lesions with lower J-CTO scores were associated with a shorter GW manipulation time and higher procedural success rates. If a given CTO lesion is associated with a high procedural success rate and a high target lesion revascularisation (TLR)-free survival rate, it may be a good candidate for PCI. Therefore, we evaluated the impact of the J-CTO score on procedural outcome and TLR after CTO recanalisation.
Methods
STUDY POPULATION AND PROTOCOL
The J-CTO Registry is a multicentre, prospective, non-randomised registry among 12 representative Japanese medical centres with experience in PCI for CTO, enrolling from April 2006 to November 2007 (Online Appendix 1). The inclusion/exclusion criteria, the definition of CTO, the angiographic analysis of the target procedures, and the study design have already been described10. The current study population consisted of 528 CTO lesions in 498 consecutive patients enrolled in the J-CTO Registry. Lesion success was defined as the final percentage of diameter stenosis of <50% assessed by quantitative coronary angiography. Procedural success was defined as an achievement of lesion success without a composite of death, Q-wave myocardial infarction (MI), and TLR before hospital discharge. Successful recanalisation of the CTO was achieved in 457 CTO lesions in 437 patients (Figure 1).
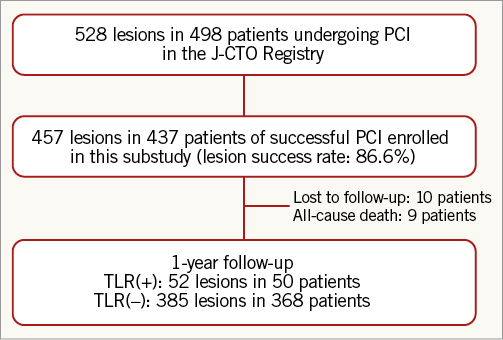
Figure 1. Patient flow chart.
The institutional review board at each participating centre approved the study protocol, and written informed consent was obtained from all the study patients.
The antiplatelet agent regimen consisted of aspirin indefinitely and thienopyridine (200 mg ticlopidine or 75 mg clopidogrel daily) for at least six months after drug-eluting stent (DES) implantation and at least one month after bare metal stent (BMS) implantation or balloon angioplasty.
Coronary angiographic analysis was performed on the angiograms obtained by scheduled follow-up angiography between eight and 12 months after PCI. If unscheduled coronary angiography was performed before eight months and resulted in TLR, the angiogram was included in the coronary angiographic analysis.
Baseline and follow-up data were recorded by clinical research coordinators from an independent clinical research organisation (Research Institute for Production Development, Kyoto, Japan) (Online Appendix 2). Follow-up data were obtained from the hospital charts or by contacting patients and/or the referring physicians.
STUDY ENDPOINTS AND DEFINITIONS
The definitions of pre-procedural and post-procedural characteristics have been previously reported10. Qualitative and quantitative angiographic analyses were performed at Tokyo Core Analysis Laboratory located in Tokai University. The definition of the J-CTO score has already been described in detail9. In brief, we selected five variables (previously failed lesion, blunt type of entry, calcification, bending, and occlusion length ≥20 mm), assigned one point to each variable, and summed up the points to obtain a total score for a lesion (J-CTO score). The J-CTO score was a grading scale of procedural difficulty predicting successful GW crossing within 30 min, and was subdivided into four groups with J-CTO scores of 0, 1, 2, and ≥3. GW manipulation time was defined as the time from the initial insertion of the GW into the coronary lumen to the successful crossing of the GW through the lesion.
Angiographic binary restenosis of the target lesion was defined as a diameter stenosis of ≥50%. The primary endpoint measure for the midterm follow-up analysis was TLR at one year. The net midterm success rate was calculated by multiplying the lesion success rate by the TLR-free survival rate. The secondary endpoint was a major adverse cardiac event comprising a composite of all-cause death, myocardial infarction (MI), and TLR. TLR consisted of repeat PCI and CABG due to restenosis or reocclusion of the target lesion. MI was defined as the presence of new pathological Q-waves on the electrocardiogram, or as an increase in creatine kinase myocardial band level to ≥3-fold the upper limit of normal. Stent thrombosis was defined on the basis of the Academic Research Consortium definitions11.
STATISTICAL ANALYSIS
Categorical variables were expressed as numbers and percentages and were compared using the chi-square test. Continuous variables were presented as mean±standard deviation or median and interquartile range. Continuous variables were compared using the Student’s t-test or the Wilcoxon rank-sum test for two-group comparisons and the Kruskal-Wallis test for four-group comparisons. Multiple lesions in a patient were assumed to be independent of each other. Cumulative incidence was determined using Kaplan-Meier curves, and differences were assessed using the log-rank test. Multivariate analysis to evaluate independent risk factors for TLR using clinical, angiographic, and procedural variables was performed using forward stepwise logistic regression analysis. We used the 33 variables listed in Table 1 and Table 2 as potential independent variables. Logistic regression analysis using the factors associated with TLR (p<0.1) was performed to determine the independent risk factors for TLR. Using a logistic regression model, we minimised the influence of the timing of TLR. Eligible lesions for logistic regression analysis included the lesions with TLR within one year and those with complete follow-up but without TLR at one year. Independent risk factors for TLR were expressed as odds ratios and 95% confidence intervals. All statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). All reported p-values were two-sided, and p-values of <0.05 were considered to be statistically significant.
Results
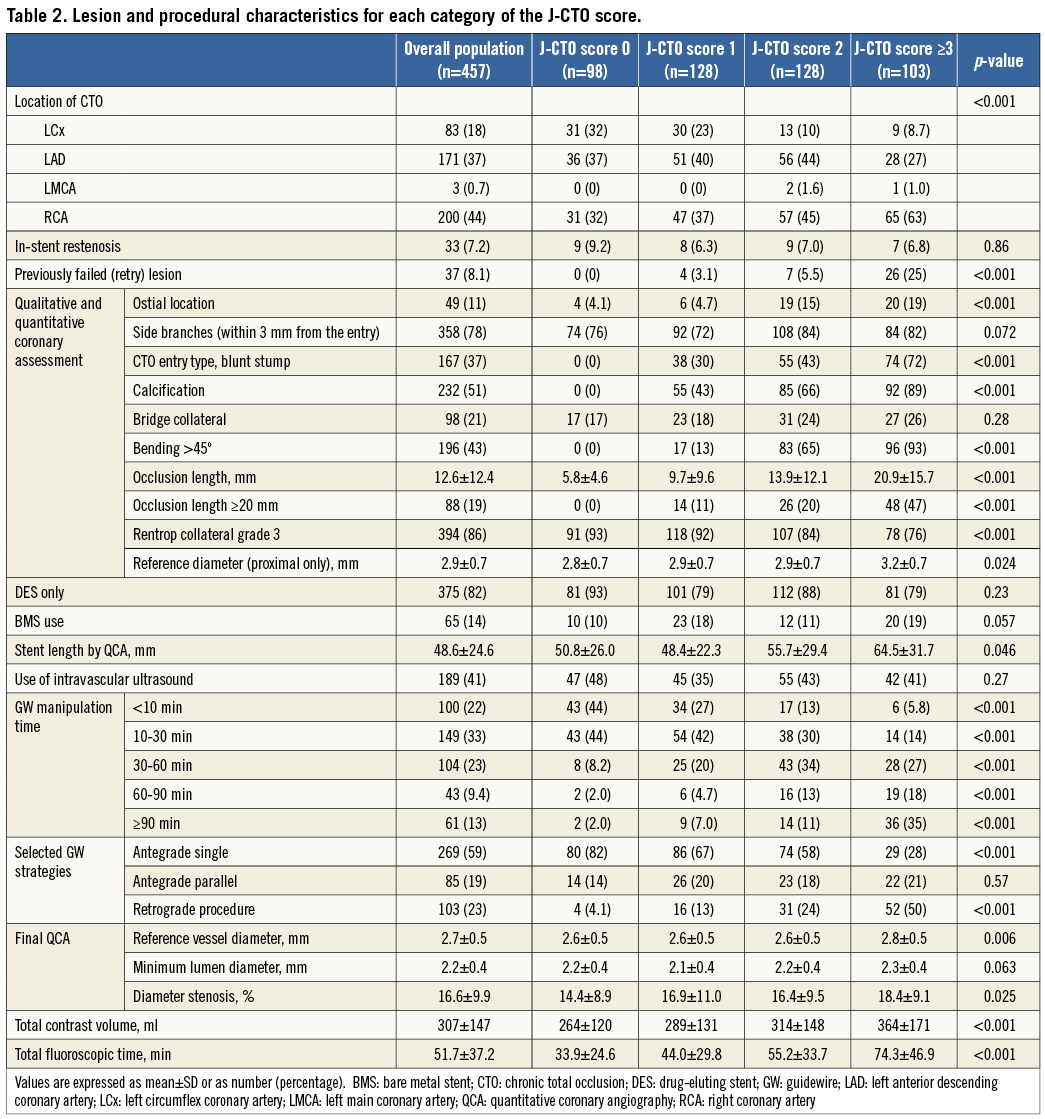
PATIENTS, LESIONS, AND PROCEDURAL CHARACTERISTICS
At baseline, categories according to the J-CTO score (J-CTO scores of 0, 1, 2, and ≥3) were distributed evenly and consisted of 98 lesions, 128 lesions, 128 lesions, and 103 lesions, respectively. Baseline patient characteristics were similar across the four categories of the J-CTO score, except for the significantly lower prevalence of multivessel disease in patients with a J-CTO score of ≥3 (Table 1). The GW manipulation time and the proportion of the retrograde approach increased proportionately with high J-CTO score categories (Table 2). The total number of each implanted DES type was as follows: sirolimus-eluting stents (697, 81%), paclitaxel-eluting stents (155, 18%), and biolimus-eluting stents (13, 2%). Out of 65 lesions implanted with BMS, six were in large vessels (reference vessel diameter >4.0 mm) and 42 were in small vessels (reference vessel diameter <2.5 mm). In this registry, the initial lesion success rates according to the J-CTO score categories of 0, 1, 2, and ≥3 were 97.0%, 92.1%, 86.5%, and 73.6%, respectively (p<0.001).
CLINICAL OUTCOMES
During the one-year follow-up period, 10 patients were lost to follow-up. The cumulative incidence of TLR was 11.4%. All-cause death occurred in 10 patients, one of whom underwent TLR 101 days later and died from a non-cardiac event 290 days later (Table 3, Figure 1). Cardiac death occurred in five patients, in two because of sudden death and in three because of unknown causes. Non-cardiac death occurred in five patients, two of whom died from stroke, one from chronic renal failure, one from renal carcinoma, and one from pneumonia. Discontinuation of thienopyridine within one year was observed in 32 (7.7%) out of the 413 patients after DES implantation, discontinuation of aspirin in two (0.5%), and both aspirin and thienopyridine in one (0.2%). Definite stent thrombosis was observed in one patient 19 days after DES implantation in spite of good adherence to dual antiplatelet therapy (Table 3).
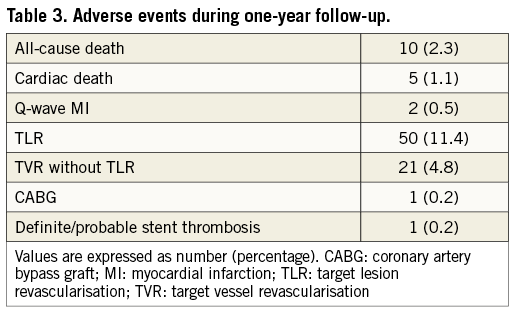
The TLR rates at one year according to the J-CTO score categories of 0, 1, 2, and ≥3 were 5.3%, 11.1%, 16.7%, and 13.4%, respectively (p=0.082) (Figure 2). The TLR rates at one year according to the five categories of GW manipulation time (<10 min, 10-30 min, 30-60 min, 60-90 min, and ≥90 min) were 7.2%, 9.9%, 13.0%, 12.2%, and 22.4%, respectively (p=0.051) (Figure 3). In the retrograde approach, the TLR rate at one year showed no significant differences compared with that in the antegrade approach alone group (16.3% vs. 10.6%, p=0.122). Combining the initial procedural lesion success rate and TLR-free survival rate of all lesions in this registry, the net midterm success rates according to the J-CTO score categories of 0, 1, 2, and ≥3 were 91.9%, 81.9%, 72.1%, and 63.7%, respectively (p<0.001) (Figure 4).
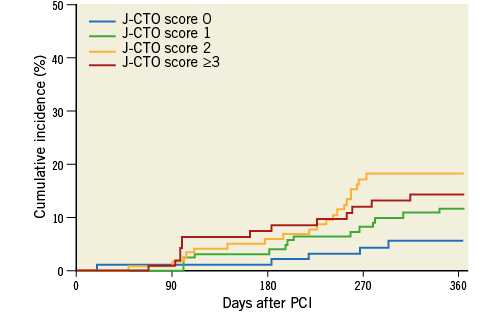
Figure 2. The cumulative incidences of TLR at one-year follow-up after successful PCI for CTO lesions according to the J-CTO score categories. CTO: chronic total occlusion; PCI: percutaneous coronary intervention; TLR: target lesion revascularisation
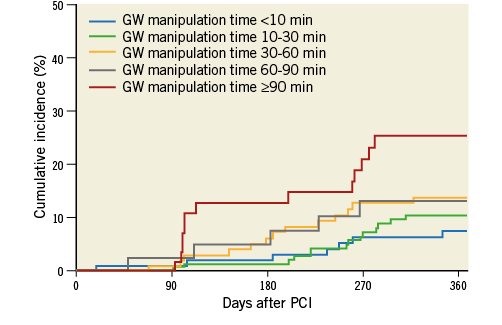
Figure 3. The cumulative incidences of TLR at one-year follow-up after successful PCI for CTO lesions according to the GW manipulation time categories. CTO: chronic total occlusion; GW: guidewire; PCI: percutaneous coronary intervention; TLR: target lesion revascularisation
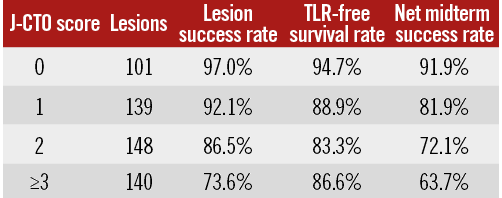
Figure 4. The differences in lesion success, TLR-free survival, and net midterm success rates across the J-CTO score categories. The net midterm success rate was calculated by multiplying the lesion success rate by the TLR-free survival rate. TLR: target lesion revascularisation
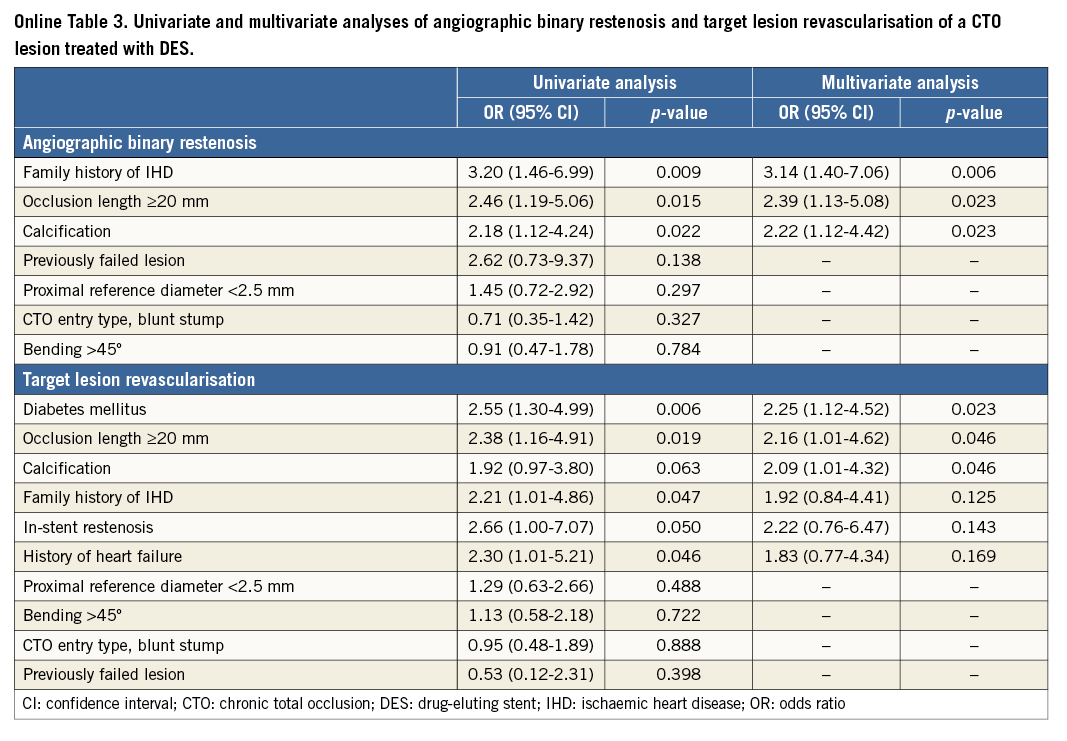
UNIVARIATE AND MULTIVARIATE RISK FACTORS FOR TLR
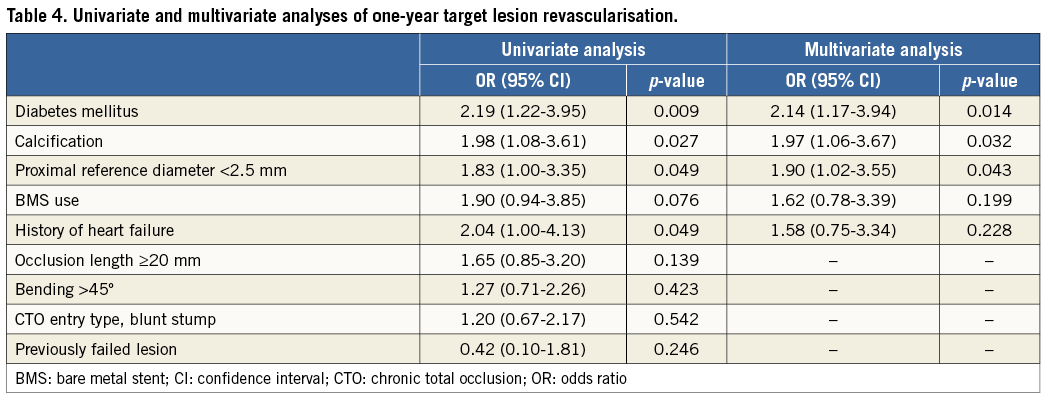
The univariate correlates of TLR included diabetes mellitus, a history of heart failure, calcification, and proximal reference diameter <2.5 mm. Using logistic regression analysis, diabetes mellitus, calcification, and proximal reference diameter <2.5 mm were identified as independent risk factors for TLR (Table 4).
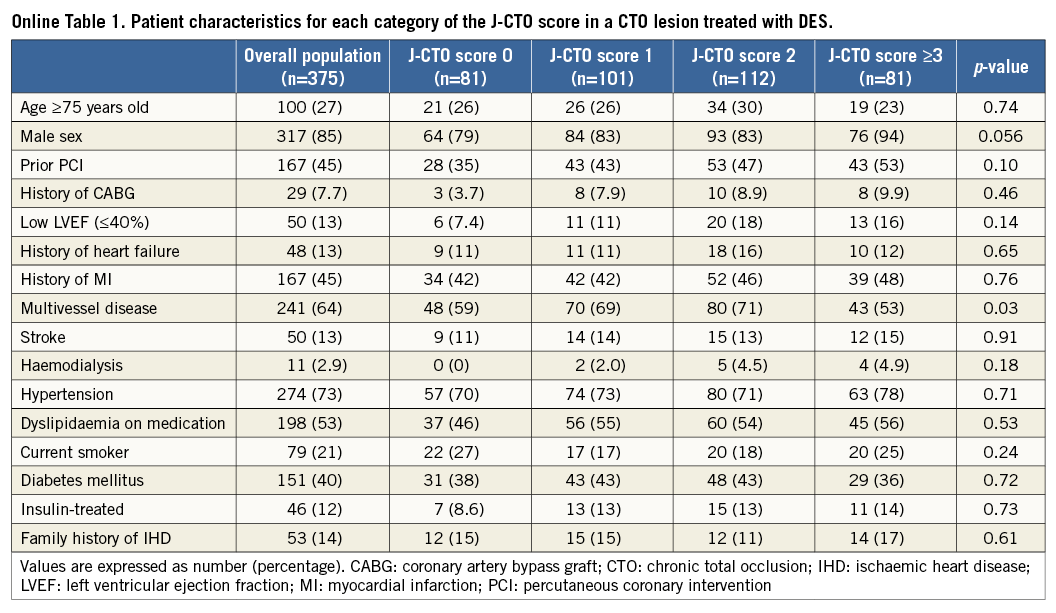
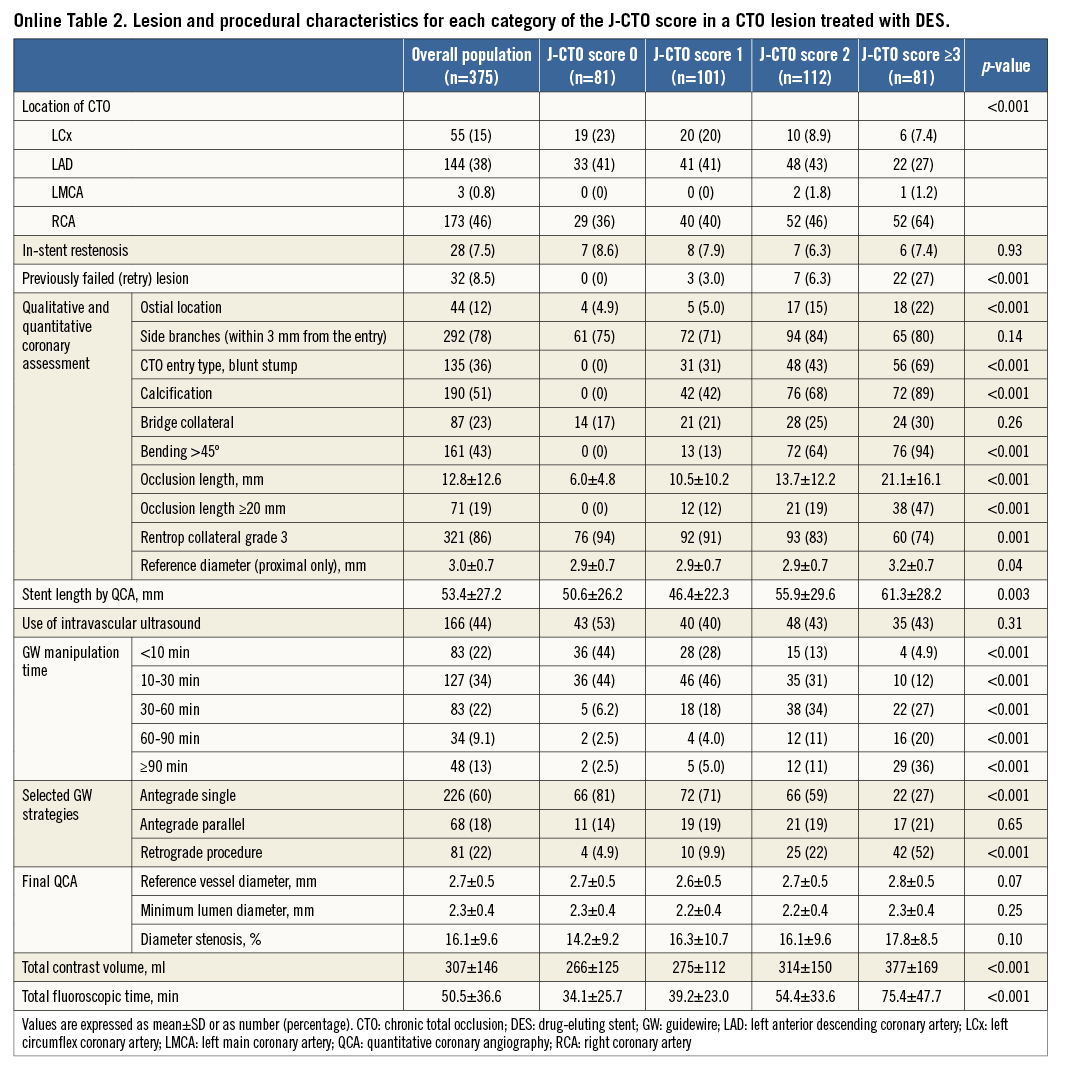
ANGIOGRAPHIC AND CLINICAL OUTCOMES OF CTO LESIONS TREATED WITH DES
Within subgroup analysis, we assessed angiographic and clinical outcomes at one year after DES implantation. Baseline characteristics are summarised in Online Table 1 and Online Table 2. Of 375 lesions after DES implantation, angiographic follow-up at ≥6 months was performed in 240 lesions in 227 patients (64.0%) at a median of 252 days (interquartile range, 235-281 days). Angiographic binary restenosis was observed in 45 lesions (18.8%). The angiographic binary restenosis rate of patients in whom intravascular ultrasound (IVUS) was used showed no significant differences compared with that of no IVUS (20.4% vs. 17.5%, p=0.618). Univariate and multivariate correlates of in-stent restenosis included family history, calcification, and occlusion length ≥20 mm (Online Table 3).
According to the J-CTO score categories of 0, 1, 2, and ≥3, the TLR rates at one year were 3.9%, 12.0%, 16.2%, and 10.3%, respectively (p=0.080). The TLR rates at one year augmented with increasing GW manipulation time. Moreover, the TLR rates at one year according to the five categories of GW manipulation time (<10 min, 10-30 min, 30-60 min, 60-90 min, and ≥90 min) were 6.2%, 9.2%, 11.4%, 11.9%, and 23.4%, respectively (p=0.037). The multivariate correlates of TLR after DES implantation included diabetes mellitus, calcification, and occlusion length ≥20 mm (Online Table 3).
Discussion
In this substudy, we investigated the impact of the J-CTO score on procedural outcome and TLR after CTO recanalisation. The main findings of this study are as follows: 1) the TLR rate augmented with increasing GW manipulation time, and 2) CTO lesions with lower J-CTO scores were associated with a high TLR-free survival rate and a high procedural success rate with a short GW manipulation time, resulting in an elevated net midterm success rate.
A meta-analysis showed that the introduction of DES, compared with BMS, has significantly decreased the restenosis rate and target vessel revascularisation after PCI for CTO12. Furthermore, Valenti et al13 reported that everolimus-eluting stents were associated with a very high patency rate, and a meta-analysis showed that second-generation DES were associated with improved angiographic and clinical outcomes compared to first-generation DES14. Kato et al15 reported that the TLR rate after sirolimus-eluting stent implantation was significantly higher in CTO lesions than in non-CTO lesions. However, there are few data on the specific factors of TLR in patients who underwent DES implantation for CTO lesions, compared with those who underwent DES implantation for non-CTO lesions. GW manipulation time is one of the procedural differences between CTO recanalisation and non-CTO: it is a unique aspect limited to CTO recanalisation due to lesion complexity. In our study, GW manipulation time correlates with lesion complexity based on the J-CTO score, and the TLR rates increased proportionately with GW manipulation time. Recently, the introduction of new strategies such as the parallel wire technique and the retrograde approach has contributed to the improvement of procedural success rates16-18. Syrseloudis et al19 reported that advanced CTO techniques and equipment have resulted in an increase in the successful treatment of highly complex lesions according to the J-CTO score, but the total success rate did not substantially improve for the increased treatment of complex lesions. Nombela-Franco et al20 reported that the J-CTO score helps to predict the complexity of CTO recanalisation but it is not associated with the final success rate. The GW manipulation time and the procedural success rate may be enhanced by the development of new devices and techniques or the operator’s experience. However, lesion complexity may affect clinical outcomes after CTO recanalisation regardless of the procedural differences. In our study, the retrograde approach was not associated with TLR despite being used for highly complex lesions. Thus, the presence of calcification and occlusion length ≥20 mm especially affected both the procedure and TLR of a CTO lesion treated with DES. If a lesion has a lower J-CTO score, it is expected to achieve both a high procedural success rate with short GW manipulation time and an increased TLR-free survival rate; however, diabetes mellitus remained an independent risk factor for TLR.
Study limitations
This study has several limitations. First, the decision to perform TLR was left to the individual operators in each participating centre, and it was largely dependent on the preferences of each patient and attending physician. Moreover, the relatively high rate of angiographic follow-up could have increased the TLR rate, which might have resulted in the risk of TLR in the proximal reference diameter <2.5 mm. Second, the clinical outcomes of the second-generation DES may differ from those of the first-generation DES which were predominantly used in this study. Finally, to predict the net long-term success beyond one year, further correction using additional factors may be needed.
Conclusions
Patients with CTO lesions with lower J-CTO scores are expected to achieve a high procedural success rate with a short GW manipulation time and an increased TLR-free survival rate, resulting in an elevated net midterm success rate; however, patients with high J-CTO scores still remain an issue.
| Impact on daily practice A lesion with a high J-CTO score still remains an issue. The presence of calcification and an occlusion length ≥20 mm especially affect both the procedure and TLR of a CTO lesion treated with DES. To lower the TLR rate for these lesions, it is necessary to consider further strategies of PCI for CTO. |
Acknowledgements
The authors are very grateful to the late Dr. Kazuaki Mitsudo for his guidance in the field of catheter intervention and would like to dedicate this paper to him. The authors would also like to thank S. Tezuka, M. Kawate, and W. Uematsu for their enthusiastic support in the preparation of this manuscript.
Funding
This study was supported by a grant from the Japan Heart Foundation, Tokyo, Japan.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Appendix 1. List of the participating centres and investigators
Hokkaido Ohno Hospital: Takehiro Yamashita; Tsukuba Medical Center Hospital: Yuichi Noguchi; Tokyo University Hospital: Toshihiro Morita; Keio University Hospital: Yasushi Asakura; Saiseikai Yokohama City Eastern Hospital: Toshiya Muramatsu; Yokohama Rosai Hospital: Kenichi Kato; Showa University Northern Yokohama Hospital: Masahiko Ochiai; Tokai University School of Medicine: Yoshihiro Morino, Toshiharu Fujii; Shizuoka Prefectural Hospital: Osamu Doi; Kyoto University Hospital: Takeshi Kimura, Takeshi Morimoto; National Hospital Organisation Kyoto Medical Center: Mitsuru Abe; Tokushima Red Cross Hospital: Yoshikazu Hiasa; Kurashiki Central Hospital: Kazuaki Mitsudo, Kazushige Kadota, Hiroyuki Tanaka; Tsuchiya General Hospital: Yasuhiko Hayashi; Miyazaki Medical Association Hospital: Yoshisato Shibata; Sequoia Hospital: Tomoaki Hinohara
Online Appendix 2. Study organisation
Steering Committee: Kazuaki Mitsudo (Principal Investigator), Toshiya Muramatsu, Takeshi Kimura, Yasuhiko Hayashi, and Kazushige Kadota.
Clinical Events Committee: Tomoaki Hinohara, Yasushi Asakura, and Masahiko Ochiai.
Data Safety Monitoring Board: Tomoaki Hinohara, Osamu Doi, and Toshihiro Morita.
Statistical Analysis: Takeshi Morimoto and Mitsuru Abe.
Coordinating Centre: Research Institute for Production Development, Kyoto, Japan. Satoshi Shizuta, Saori Tezuka, Mika Kawate, and Wakako Uematsu.
Angiographic Core Laboratory: Tokyo Core Analysis Laboratory, Tokai University, Isehara, Japan