
Abstract
Aims: Correction of mitral and/or tricuspid regurgitation (MR, TR) frequently leads to poor outcomes in the days following intervention. We sought to understand how abrupt correction of MR and TR affects ventricular load and to investigate if gradual correction is beneficial.
Methods and results: MR and TR were simulated using the CircAdapt cardiovascular system model with effective regurgitant orifice (ERO) areas of 0.5 cm2 and 0.7 cm2. Ventricular and atrial contractility reductions to 40% of normal and pulmonary hypertension were simulated. Abrupt and gradual ERO closure were simulated with homeostatic regulation of blood pressure and volume. Abrupt correction of MR increased left and right ventricular fibre stress by 40% and 15%, respectively, whereas TR correction increased left and right ventricular fibre stress by 26% and 19%, respectively. This spike was followed by a rapid drop in fibre stress. Myocardial dysfunction prolonged the spike but reduced its amplitude. Right ventricular fibre stress increased more with pulmonary hypertension and TR. Gradual correction demonstrated no spike in tissue load.
Conclusions: Simulations demonstrated that abrupt ERO closure creates a transient increase in ventricular load that is prolonged by worsened myocardial condition and exacerbated by pulmonary hypertension. Gradual closure of the ERO abolishes this spike and merits clinical investigation.
Introduction
Chronic mitral regurgitation (MR) and tricuspid regurgitation (TR) cause progressive ventricular and atrial enlargement through altered protein expression, sarcomere remodelling, and myocyte elongation that creates a “vicious cycle” of dilatation and worsening regurgitation1,2,3,4. Due to compensatory remodelling, patients are frequently asymptomatic5. When indicated, repair or replacement of the valve aims to restore competency, abruptly terminating retrograde flow6. If indicated, tricuspid valve repair or replacement is generally performed concomitant with left-sided valve intervention7. Long-term residual regurgitation due to incomplete repair or paravalvular leakage allows the cycle of dilatation and regurgitation to restart and is associated with worse long-term outcomes8,9.
Patients undergoing mitral valve replacement for chronic regurgitation experience longer stays in intensive care including extracorporeal life support, use of inotropes, and mechanical ventilation than for other cardiac interventions at similar levels of risk such as aortic valve replacement10,11. In-hospital mortality from mitral valve replacement surgery has recently been reported at 5.5% with 63.3% morbidity12. We hypothesised that abrupt changes in ventricular loading conditions in patients with chronic regurgitation following valve replacement may be harmful in the short term and contribute to post-procedural myocardial dysfunction and mortality. We additionally hypothesised that gradual correction of regurgitation may be beneficial by gently unloading the heart while fully resolving regurgitation. A proposed modification to prosthetic valves to allow gradual correction is shown in Figure 1. To investigate these hypotheses in a controlled manner, we simulated correction of MR and TR in a validated cardiovascular system model and compared the effects of abrupt and gradual correction of regurgitation on cardiac pump function and tissue mechanics.
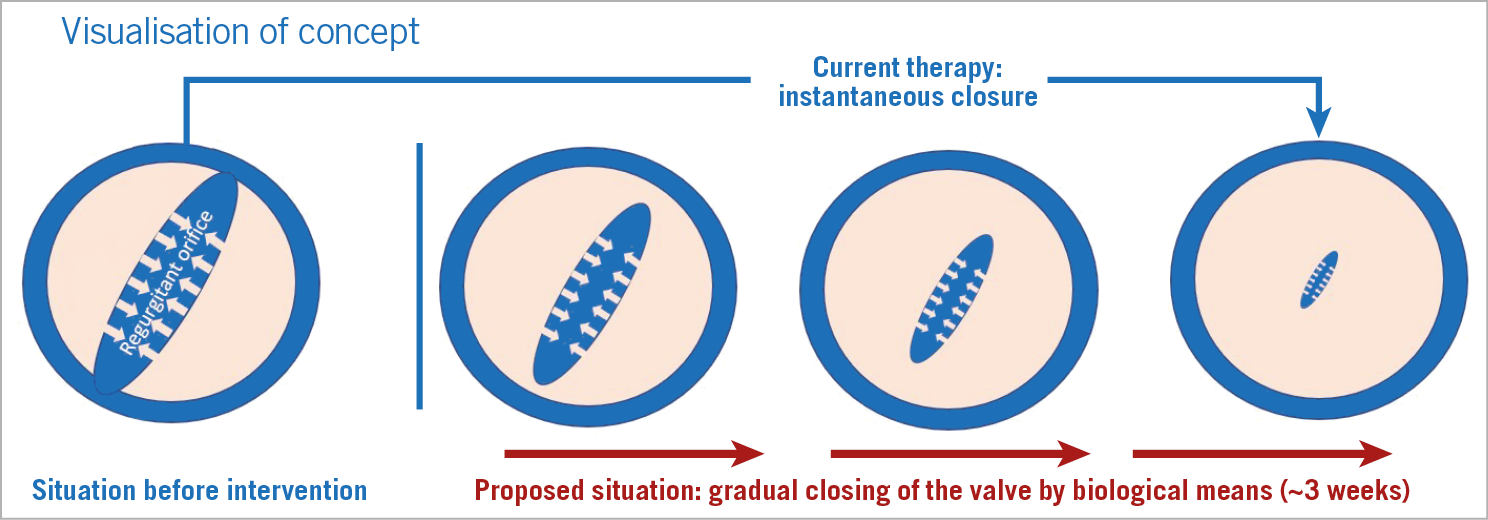
Figure 1. Gradual reduction of regurgitation. We envisage a transiently leaking prosthetic valve with the following characteristics. The valve would admit a regurgitant flow on implantation. Over the following weeks, the regurgitation diminishes until complete closure is achieved through a degrading biogel on the valve leaflets that impedes closure.
Methods
THE CIRCADAPT MODEL
CircAdapt is a well-established lumped-parameter model of the heart and circulation that enables realistic simulation of beat-to-beat cardiovascular mechanics and haemodynamics (Figure 2A),13,14,15. Valvular flows in health and disease have been validated through direct comparison both with clinical Doppler recordings and with derived echocardiographic parameters14,16. Ventricular tissue mechanics and pump function have been extensively and quantitatively validated against experimental and clinical measurements, including myocardial strain from tagged magnetic resonance data and echocardiography15,17,18. Furthermore, CircAdapt enables simulation of myocardial structural adaptation to chronic alterations in mechanical load due to exercise training or pathology19. Adaptation alters the thickness, area, and stiffness of heart and blood vessel walls in the systemic and pulmonary circulations to normalise local tissue loading. All simulations in this paper use source code downloadable from www.circadapt.org15. Detailed descriptions of the modules below are provided in Supplementary Appendix 1. A simulation flow chart is shown in Supplementary Figure 1.
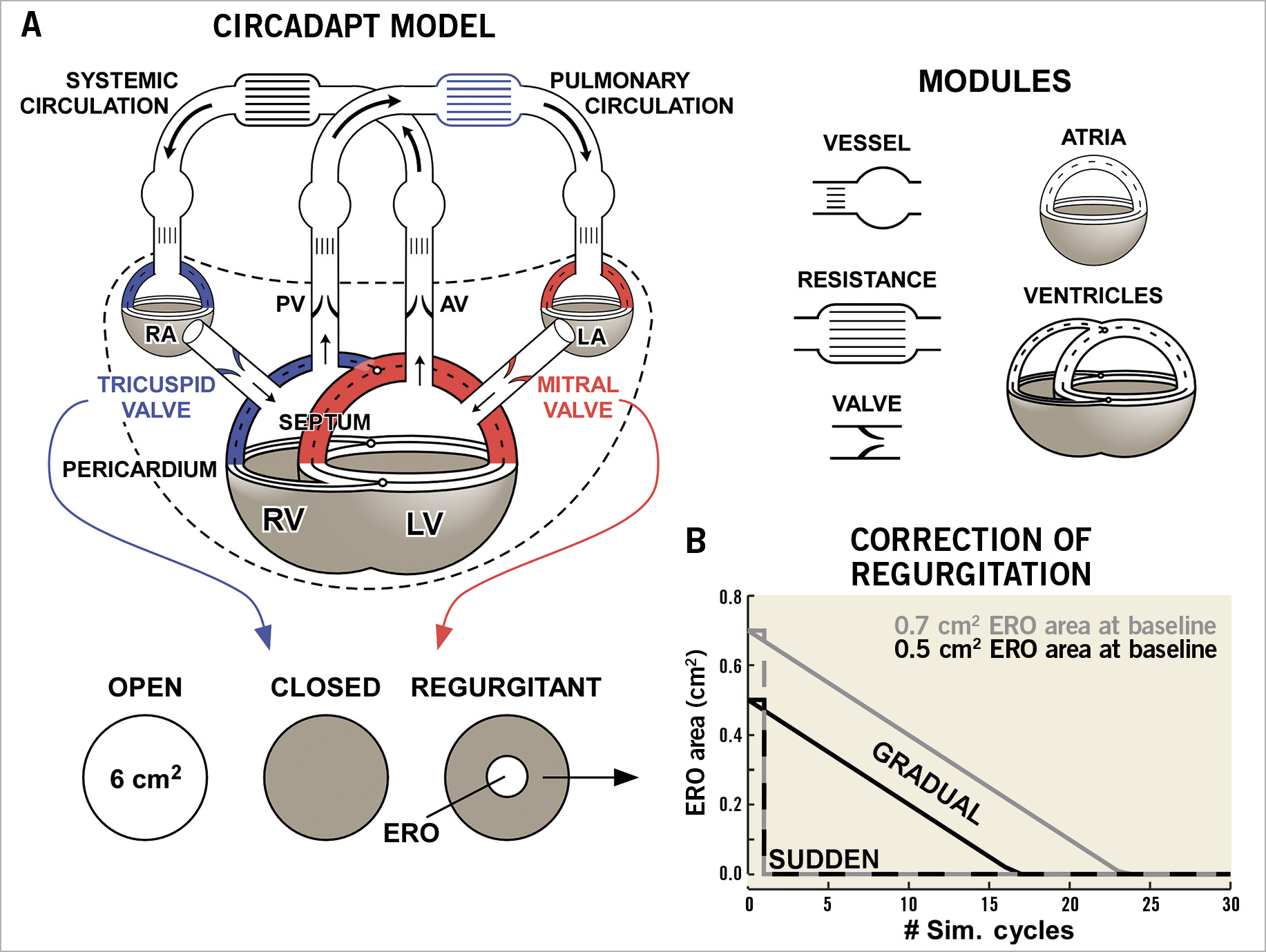
Figure 2. Computational methods. A) The structure of the CircAdapt cardiovascular system model. The left ventricle (LV), left atrium (LA), and mitral valve are highlighted in red. The right ventricle (RV), right atrium (RA), tricuspid valve, and pulmonary circulation are highlighted in blue. Modified from Lumens et al with permission14. B) The course of closure of a valve ERO area of 0.5 cm² (black) and 0.7 cm2 (grey) for sudden closure (dashed lines) and gradual closure (solid lines).
HOMEOSTATIC CONTROL
Circulating blood volume is used to maintain mean arterial pressure (MAP) and cardiac output (CO) through homeostatic control, representing venous pooling and fluid retention or excretion by the renin-angiotensin-aldosterone system. When homeostatic control is enabled, the ratio of the current MAP to the target MAP is calculated after each simulation of a single cardiac cycle, as is the ratio of the current CO to the target CO. Systemic vascular resistance increases when MAP is too low and decreases when CO is too low, mimicking systemic arteriolar constriction or dilation. Blood is injected or removed from the systemic circulation based on the ratio of MAP. Subsequent simulation cycles repeat this process until the target MAP and CO are reached. Each cycle with homeostatic control enabled represents a snapshot into a process that takes hours to days, and that depends on renal function amongst other factors.
VALVULAR FUNCTION
The valve module simulates blood flow between two cardiac chambers, or a chamber and a large vessel, where energy losses can occur. The valve is a narrow orifice whose area varies over time during a cardiac cycle, described by the Bernoulli equation. We assume unsteady, incompressible and non-viscous laminar flow. We assume that no pressure is regained after the valve despite the velocity decrease, representing energy lost through friction or turbulence. In simulations of MR or TR, a “closed” valve has an effective regurgitant orifice (ERO) allowing backflow (Supplementary Figure 2).
FIBRE STRESS
A phenomenological model describes the dynamic relationship between myofibre stress and strain and determines cavity pressure by Laplace’s law14,15. The active stress component incorporates length dependence of the force generated and the duration of contraction (Supplementary Appendix 1).
BASELINE SIMULATIONS
For all simulations, the opening areas of the mitral and tricuspid valves were set to 6 cm2, and the aortic and pulmonary valves were set to 3.5 cm2. We started with a healthy reference simulation15. The ERO area was set to 0.3 cm2 in the mitral or tricuspid valve to simulate compensated MR or TR, respectively. Using homeostatic control, CO was maintained at 5.1 l/min and MAP was maintained at 90 mmHg with a heart rate of 70 bpm. We then simulated tissue adaptation in response to chronic regurgitation, as described previously19. The resulting simulations represent a compensated and well-tolerated mild regurgitation.
DECOMPENSATED REGURGITATION
Decompensated mitral or tricuspid regurgitation is simulated by taking the compensated MR or TR simulations and increasing the ERO area to 0.5 cm2 and 0.7 cm2 in the mitral or tricuspid valve. Heart rate is maintained at 70 bpm but, to represent the compromised haemodynamic status of patients with decompensated regurgitation requiring surgery, CO is reduced to 3.6 l/min and MAP to 75 mmHg.
MYOCARDIAL DYSFUNCTION
To represent myocardial failure following decompensation, we simulated varying degrees of myocardial dysfunction on the left and/or right side of the heart. On the left side, the left ventricular (LV) free wall, septum, and left atrial (LA) myocardium were dysfunctional, and on the right side the right ventricular (RV) free wall and right atrial (RA) myocardium were made dysfunctional. Both atria and ventricles were affected because chronic regurgitation is associated with atrial remodelling20,21. Myocardial dysfunction was simulated by replacing 0-40% of the wall volume with non-contractile fibrotic tissue with 3× the stiffness of healthy myocardium. Haemodynamics were stabilised by homeostatic control.
PULMONARY HYPERTENSION
TR commonly occurs secondarily to pulmonary hypertension (PH)22. We therefore repeated all TR simulations with PH present by tripling the resistance of the pulmonary circulation (Supplementary Appendix 1).
ABRUPT AND GRADUAL CORRECTION OF REGURGITATION
Abrupt correction of regurgitation was simulated by setting the ERO area of the mitral or tricuspid valve to zero once a cardiac cycle completed. Gradual closure was simulated by reducing the ERO area by 0.03 cm2 per homeostatic control cycle until it reached zero (Figure 2B). In both cases, homeostatic control remained active and gradually altered systemic vascular resistance and blood volume to return to the same CO and MAP as before the intervention. Each simulation ran for 30 homeostatic control cycles to allow haemodynamic stabilisation. The maximum myofibre stress in each simulation cycle was used to quantify the change in myocardial load following correction of MR or TR.
Results
BASELINE SIMULATIONS OF MR AND TR
Haemodynamics at baseline are shown in Figure 3. Backflow occurs across the mitral valve during LV systole in the simulations with MR. Forward flow across the mitral valve during LV diastole is also increased, as shown by increased E- and A-wave magnitude. The retrograde flow with MR increases with ERO area (Figure 3A, top left). Pressure-volume loops show loss of LV isovolumetric contraction and relaxation phases in the simulations with MR (Figure 3A, centre). Filling pressures are not increased in the compensated MR simulation but are increased in the decompensated simulations. The simulation with reduced LV and LA myocardial function also has LV dilation (Figure 3A, centre) and increased RV systolic pressure secondary to LV failure (Figure 3A, right).
In the TR simulations, backflow occurs across the tricuspid valve during RV systole (Figure 3B, bottom left), causing RV dilation loss of RV isovolumetric contraction and relaxation (Figure 3B, right). In the simulations with RV and RA myocardial dysfunction, RV filling pressures increase (Figure 3B, right), and RV dilation becomes more pronounced. PH further dilates the RV, causing LV compression. Baseline characteristics are shown in Supplementary Table 1. LV ejection fraction (EF) and RVEF remain high in the MR and TR simulations, respectively, even when myocardial dysfunction is present.
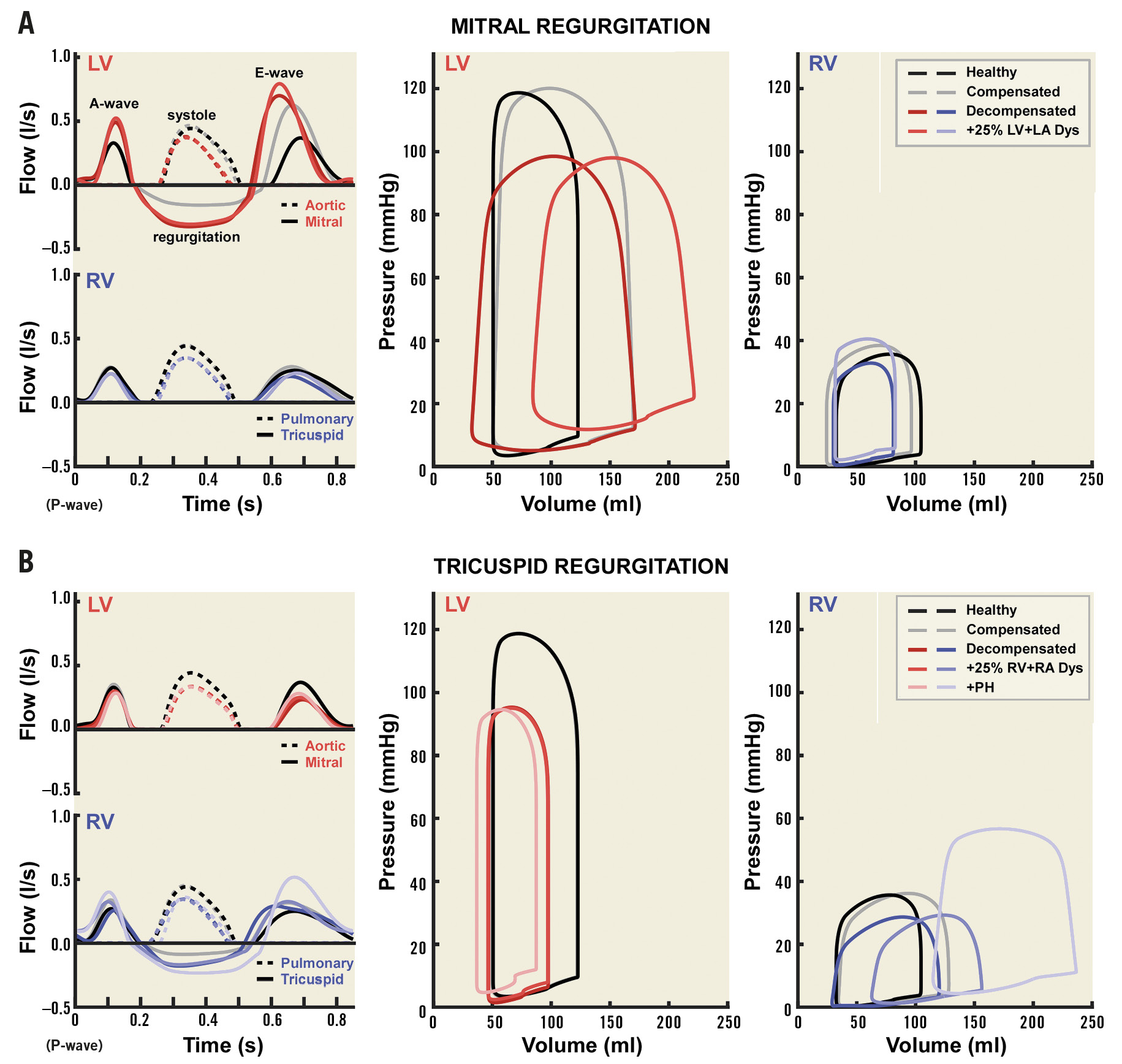
Figure 3. Baseline haemodynamics. Ventricular haemodynamics at baseline are shown for mitral regurgitation (A) and tricuspid regurgitation (B). The left panels show flow patterns across the aortic and mitral valves (top) and pulmonary and tricuspid valves (bottom). The remaining panels show pressure-volume loops for the LV (centre) and RV (right). The healthy (black) and compensated (grey) simulations are shown in all panels. Panel A also shows the 0.7 cm2 ERO area decompensated (dark) and +25% LA and LV myocardial dysfunction (light) simulations. Panel B also shows the 0.7 cm2 ERO area decompensated (dark), decompensated with 25% RA and RV myocardial dysfunction (medium), and PH with 25% RA and RV myocardial dysfunction (light) simulations.
HAEMODYNAMIC ALTERATIONS FOLLOWING CORRECTION OF REGURGITATION
Dramatic changes in LV pressure-volume loop morphology occur immediately after abrupt correction of MR (Figure 4A), with a pronounced increase in ventricular work (loop area) and a spike in peak LV systolic pressure from 102 mmHg to 135 mmHg. A reduction in ventricular work then occurs rapidly (red dashed lines). In contrast, gradual closure of the RO leads to a gradual transition in pressure-volume loop morphology and gradual reduction in ventricular work (Figure 4B). Both simulations reach the same endpoint (solid red lines). Similar behaviour is observed in RV pressure-volume loop morphology following closure of the regurgitant tricuspid valve (Supplementary Figure 3).
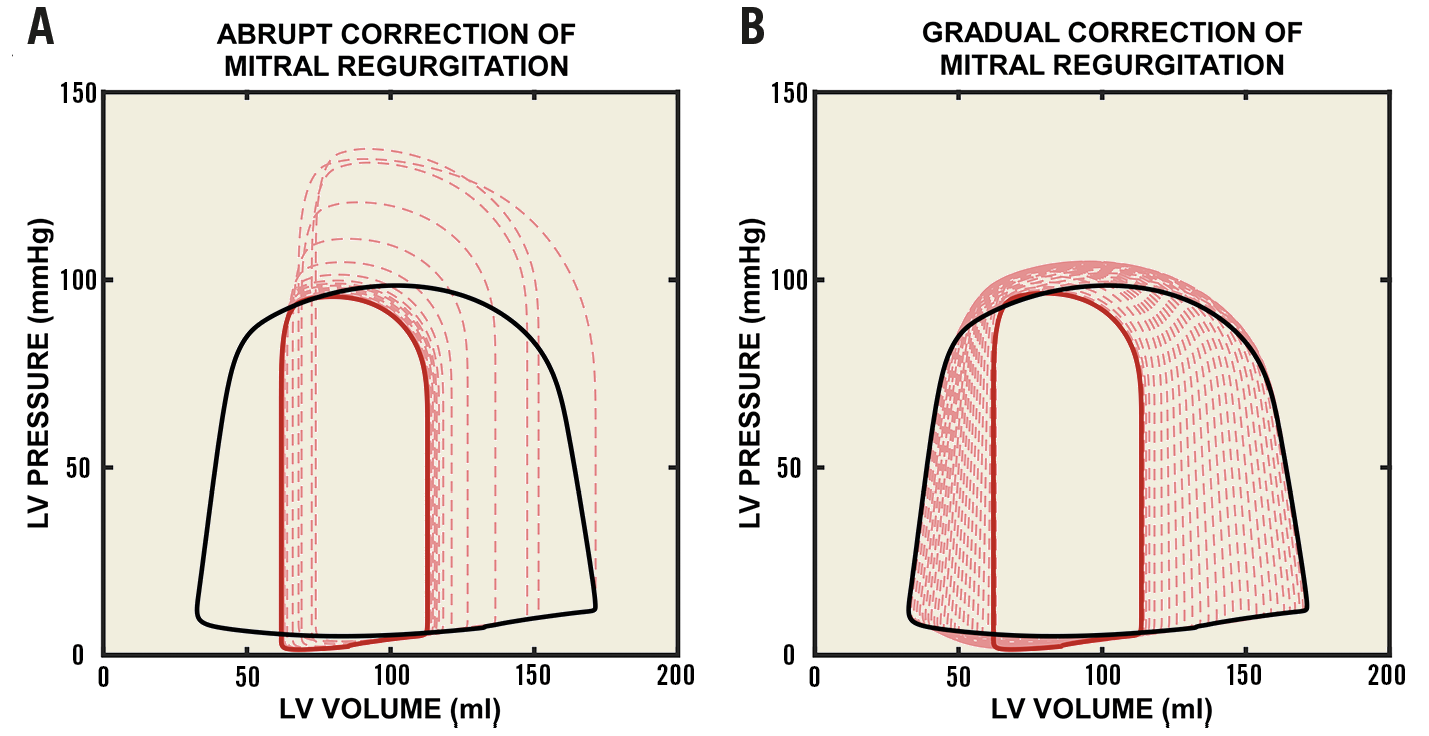
Figure 4. Effect of correction of mitral regurgitation on LV pressure-volume relationships. A) The effect of sudden closure of the ERO in the mitral valve. B) The effect of gradual closure of the ERO. The baseline pressure-volume loop is shown in black with an ERO area of 0.7 cm2. The pressure-volume loop after closure and haemodynamic stabilisation is shown in red (solid line). Intermediate pressure-volume loops are also shown (dashed lines).
A summary of characteristics following haemodynamic stabilisation after correction of MR or TR is shown in Supplementary Table 2. EF decreases after repair of regurgitation. Following correction of MR, LVEF decreases from 54% to 26% in the decompensated simulations with 0.7 cm2 ERO area at baseline and 40% LV and RV myocardial dysfunction. In the equivalent simulations with TR, the RVEF decreased from 46% to 29% following correction.
INFLUENCE OF REGURGITATION SEVERITY ON VENTRICULAR STRESS
Abrupt closure of a regurgitant valve causes a spike in LV and RV fibre stress, as shown in Figure 5. The magnitude of the increase in fibre stress depends on the severity of regurgitation prior to intervention. Figure 5A shows LV and RV peak fibre stresses for each simulation cycle following abrupt correction of MR with an ERO area at baseline of 0.7 cm2 (black dots). LV fibre stress peaks at 71.7 kPa following abrupt correction. There is a larger increase in LV and RV fibre stresses immediately following abrupt correction of MR with the larger ERO area at baseline (Figure 6, left). LV fibre stress increases by 20.5 kPa (40%) with ERO area 0.7 cm2 at baseline as compared to 14.5 kPa (29.7%) with ERO area 0.5 cm2 immediately after abrupt closure, while RV fibre stress increases by 3.6 kPa (14.8%) as compared to 1.9 kPa (8.1%). Gradual correction of MR abolishes the sudden spike in LV and RV fibre stress (Figure 5A, white dots), increasing LV and RV fibre stress by a maximum of 2.4 kPa (4.6%) and 0.4 kPa (1.6%), respectively (Figure 6, left).
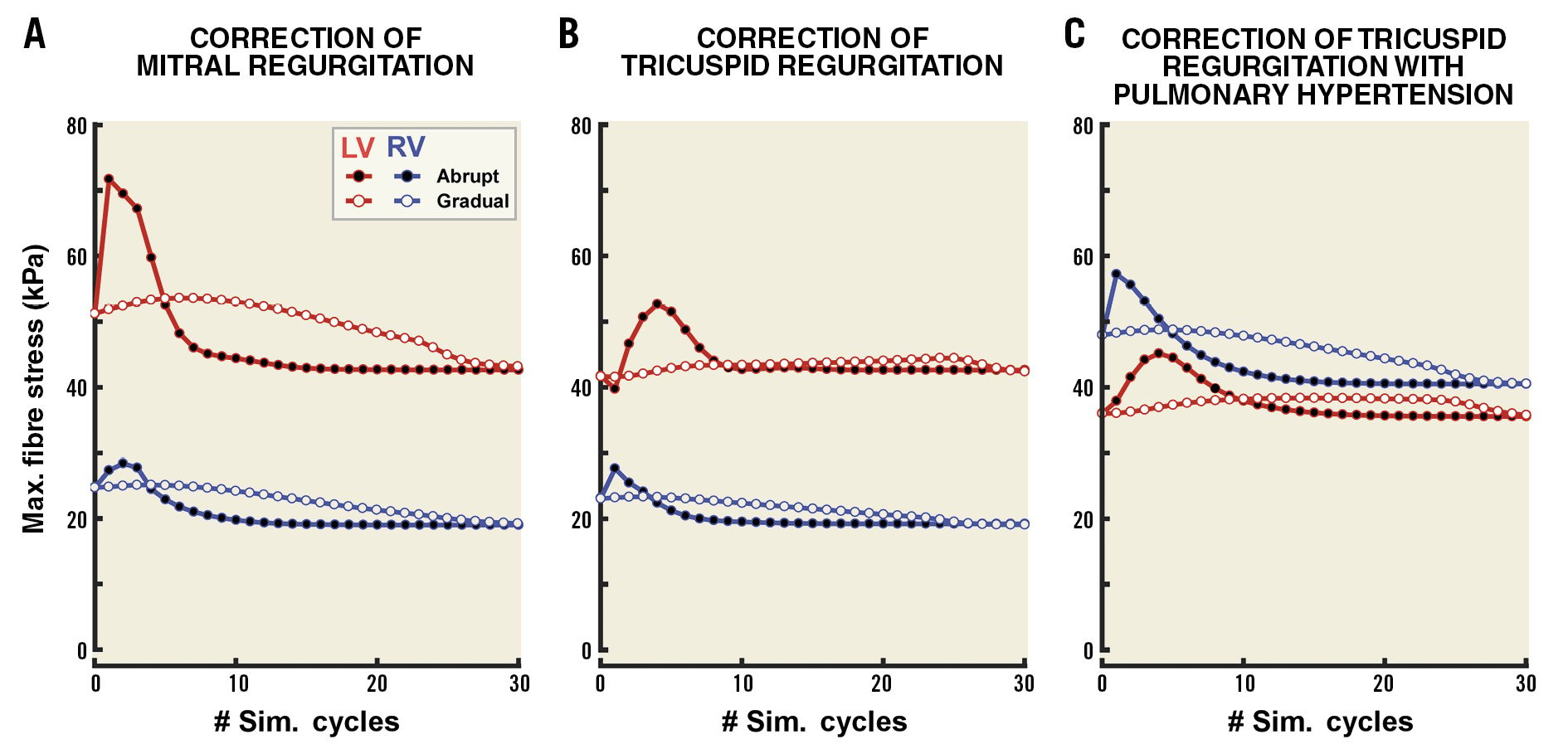
Figure 5. Effect of regurgitation severity and pulmonary hypertension on fibre stress following correction of regurgitation. The time course of LV (red) and RV (blue) peak fibre stress following correction is shown for MR (A), TR (B) and TR with PH (C). ERO area was 0.7 cm2 at baseline. Black dots indicate sudden correction and white dots indicate gradual correction.
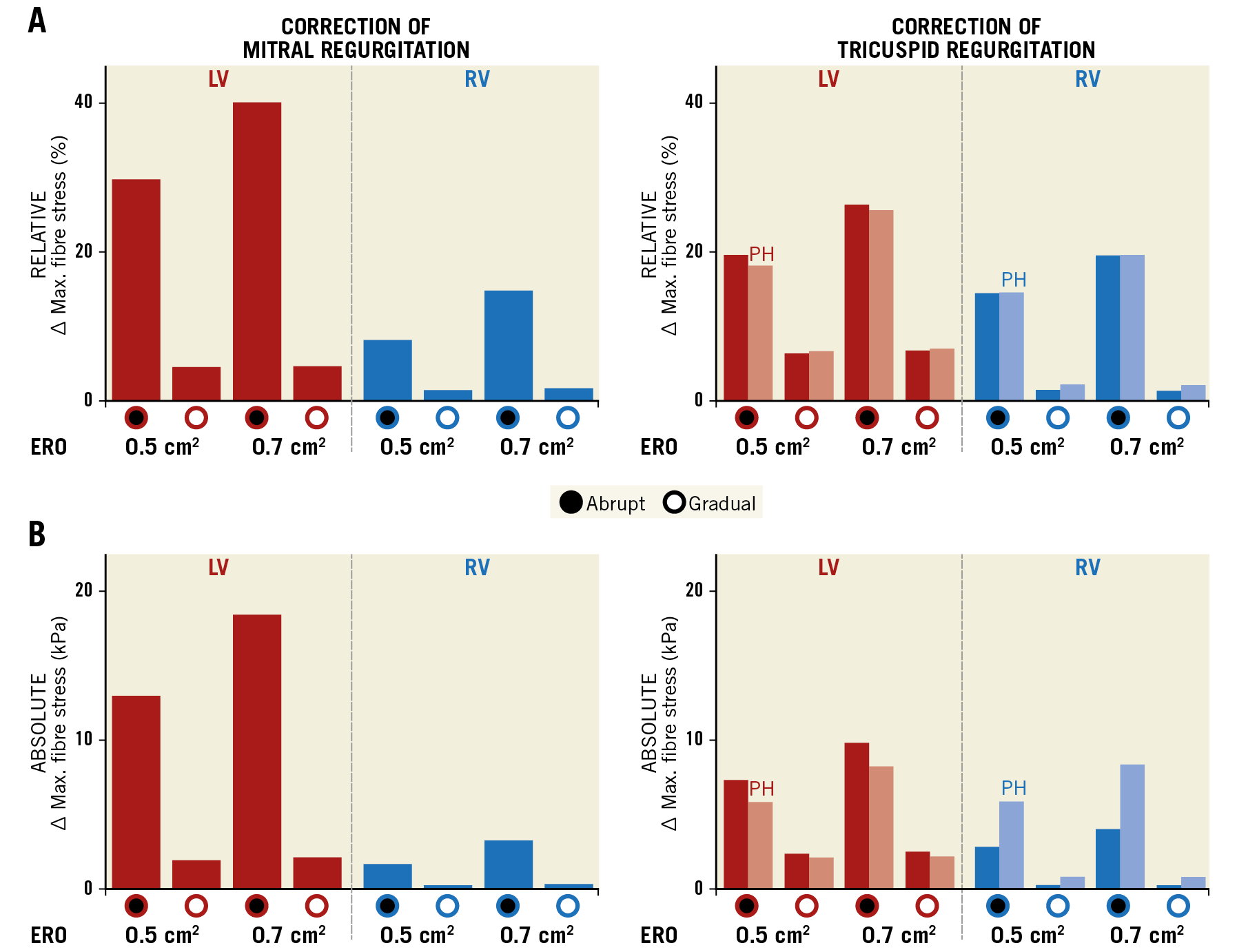
Figure 6. Severity of regurgitation affects fibre stress following correction of regurgitation. A) The maximum percentage change in fibre stress from baseline following correction for the simulations with 0.5 cm2 and 0.7 cm2 ERO area at baseline. The effect of PH is also shown for tricuspid regurgitation (lighter bars). B) The same data as absolute values.
Abrupt correction of TR leads to sharp increases in both LV and RV fibre stress that are abolished with gradual correction. However, the effects of correction of TR on ventricular stresses are less pronounced than those observed for MR (Figure 5B). As with correction of MR, a larger baseline regurgitation caused a greater increase in LV and RV fibre stress (Figure 6, right). Increased RV fibre stress immediately after correction accompanies a slight decrease in LV stress (Figure 5B). Redistribution of blood from left to right subsequently leads to an increase in LV fibre stress. In the simulations with 0.7 cm2 ERO area at baseline, sudden closure causes an increase in RV fibre stress of 4.5 kPa (19.5%), as compared with an increase of 3.2 kPa (14.4%) with 0.5 cm2 at baseline (Figure 6, right). The increase in peak RV fibre stresses with gradual closure was minor (<0.5 kPa, <2%).
PH increases RV maximum fibre stress prior to correction of TR (Figure 5C). The relative change in fibre stress after correction is similar (19.2%) to the non-PH case (Figure 6A, right). However, because of the increased RV fibre stress at baseline, the amplitude of the fibre stress spike following sudden correction of TR increases when PH is present, up to 9.2 kPa with an ERO area of 0.7 cm2 at baseline (Figure 6B, right).
INFLUENCE OF MYOCARDIAL DYSFUNCTION ON VENTRICULAR STRESS
Supplementary Figure 4 shows the influence of myocardial dysfunction on peak systolic fibre stress following correction of MR and TR. The spike in LV peak systolic stress is present in all simulations with sudden correction of MR but is absent in the gradual closure simulations. In simulations with more extensive myocardial dysfunction, the spike in systolic stress is lower but longer lasting than in simulations with healthier myocardium (Supplementary Figure 4A, left). RV peak fibre stress increases more dramatically with myocardial dysfunction than LV peak fibre stress (Supplementary Figure 4B). This elevated peak stress is still present, although lower, after correction of MR. A small spike in RV peak fibre stress is present in all simulations with sudden correction of MR (Supplementary Figure 4B, left), and is absent in the gradual correction of MR simulations (Supplementary Figure 4B, right). Sudden and gradual corrections of TR with myocardial dysfunction are qualitatively similar to corrections of MR. A more sudden spike in RV systolic fibre stress is observed after correction of TR than MR. A slight dip followed by a sharp rise in LV peak systolic fibre stress occurs following sudden correction of TR, and both are abolished by gradual correction.
Discussion
The main findings of this simulation study are that: i) abrupt cessation of MR or TR causes a sudden increase in ventricular myocardial stress, and ii) this increase in stress is preventable by gradual reduction of regurgitation. Our findings suggest that acute alterations in ventricular loading may contribute to post-procedural contractile dysfunction in patients following replacement of the mitral and tricuspid valves. Hence, valvular replacement should be adapted to achieve gradual termination of regurgitation.
Earlier studies demonstrated that mitral valve surgery can induce LV dysfunction23,24,25. Apparent induction of LV dysfunction following surgery partially reflects the inadequacies of EF as a measure of cardiac function in the presence of regurgitation26. EF is by definition higher when regurgitation occurs due to the additional backflow into the atrium increasing the apparent stroke volume. Our study shows a decrease in LVEF or RVEF following termination of mitral or tricuspid regurgitation, respectively, as this backflow is abolished. However, our results also show that an abrupt termination of this backflow causes a sudden increase in myocardial stress. Apparent ventricular dysfunction after surgery may therefore be explained by unmasking of impaired myocardial function that was hidden by regurgitation. However, the association between post-procedural dysfunction and mortality suggests that dysfunction may also result from intervention25.
Clinical data on wall stress following mitral valve surgery are limited. An acute increase in end-systolic wall stress has been reported in patients during MitraClip® (Abbott Vascular, Santa Clara, CA, USA) implantation procedures, despite decreases in end-diastolic wall stress27. An acute increase in systolic pressure was also observed. An earlier study on mitral valve replacement also showed increased end-systolic stress in patients who had chordae tendineae transected during surgery, but not in those with chordal sparing28. Our results suggest that these increases in systolic stress are caused by sudden termination of regurgitation.
Myocardial gene expression alters in response to chronic alterations in ventricular load that accompany chronic regurgitation2. Furthermore, atrial volume overload leads to atrial enlargement, fibrosis, and fibrillation20,21. While simulations cannot represent the full complexity of remodelling in chronic MR and TR, remodelling occurs gradually over a period of weeks, months, or even years in asymptomatic patients29. In contrast, current strategies for repair or replacement immediately alter ventricular loading27,28. Consequently, in severely remodelled myocardium, the abrupt change in volume load may cause additional dysfunction even after the initial spike in fibre stress, as the changes in volume loading may be too great for physiological remodelling processes to compensate in the short term. This is of particular importance in transcatheter valve replacement procedures where transient ventricular assistance is not possible.
Chronic ischaemic MR has significant mortality and morbidity following valvular surgery, and there is currently little evidence of a survival benefit from reducing secondary MR30. Patients with dilated ventricles and a disproportionate degree of secondary MR may benefit from intervention. Simulations showed that the course of LV tissue load after abrupt correction of MR or TR depends on the severity of global contractile dysfunction, with a lower but prolonged increase in peak tissue stress when myocardial contractile function was impaired. The load increase was greater in simulations with a larger ERO area. This finding may be explained by the decreased ability of “failing” myocardium to contract forcefully enough to cope with increases in ventricular load. Whether these results also apply to hearts with regional myocardial dysfunction (e.g., due to ischaemic heart disease) remains unknown and requires further research. It has been suggested that a benefit of repair over replacement may be, paradoxically, that residual regurgitation is more common following repair of ischaemic mitral regurgitation, reducing the short-term impact of surgery on the heart31. Our results support this hypothesis by demonstrating that abrupt termination of regurgitation imposes large increases in stress on the heart, which may be reduced by a persistent regurgitation. However, residual regurgitation following valve replacement is associated with worse long-term outcomes10. Our finding supports a gradual approach to complete closure, combining short-term and long-term benefits.
The amplitude of the stress increase after sudden correction of TR was exacerbated by the presence of PH, a common cause of TR22. Since correction of TR increased LV fibre stress, a gradual approach to correction of TR concomitant with MR can reduce the impact on both ventricles. A gradual approach to tricuspid replacement may be particularly beneficial when RV afterload is elevated due to conditions such as chronic obstructive pulmonary disorder or pulmonary arterial hypertension. Since PH exacerbated the spike in ventricular load, afterload reduction could also have a role in limiting the impact of abrupt termination of regurgitation on both the LV and the RV.
Limitations
Although only clinical studies can capture the complexity of a real intervention in patients, computational models allow understanding of basic pathophysiological mechanisms under tightly controlled conditions that are not possible in the clinic. Creating such conditions in animal models is expensive, raises ethical issues, and does not guarantee generalisability to humans. Hence, we established the validity of our concept in silico. Homeostatic control in CircAdapt is phenomenological. The thirty cycles shown represent a process occurring over hours or days in humans, meaning that the time for gradual closure should last well beyond the replacement procedure itself. A more complex regulation model is required to determine how quickly the regurgitant orifice should be closed. Although our findings are applicable to surgical valve replacement, our simulations best represent off-pump transcatheter valve replacement, which is feasible in high-risk severe MR patients not eligible for surgery32. The transcatheter approach might benefit more from the presented concept because the interventions available to maintain cardiac function during and immediately post procedure are more limited than for surgical approaches.
Conclusions
Simulations demonstrated that correcting MR or TR causes a spike in ventricular stress that may exacerbate postoperative cardiac dysfunction and explain the need for post-procedural inotropic support. By avoiding dramatic increases in ventricular stress, gradual correction reduces ventricular overload and may allow gradual reverse remodelling to occur. Our in silico proof-of-principle study supports designing future transcatheter prosthetic valves to allow gradual correction of regurgitation.
|
Impact on daily practice Current interventions to correct MR or TR are likely to cause a large transient increase in ventricular load that may be harmful in the short term, despite their longer-term benefits, explaining the relatively challenging course of these patients in the days after the procedure. Future transcatheter interventions for valve replacement may therefore allow a gradual reduction of regurgitation that prevents sudden alterations in ventricular loading and allows a more gradual reverse remodelling to occur. |
Conflict of interest statement
J. Lumens and J. Walmsley report research grants from Medtronic plc. R. Cornelussen and U. Wolfhard are Medtronic plc employees. R. Cornelussen and U. Wolfhard are co-inventors on patents related to the concept. P. Squara reports funding from Medtronic plc for patent maintenance fees. J. Walmsley & J. Lumens: consultancy fees from Medtronic plc.

