Abstract
The surgical treatment of isolated and concomitant tricuspid valve disease, especially functional tricuspid valve regurgitation, remains controversial. Functional tricuspid regurgitation may be classified into defined stages, and surgical treatment may be tailored to the extent of the disease. This report describes current surgical techniques for tricuspid valve surgery and their results.
Introduction
The tricuspid valve remains undertreated, even though severe tricuspid regurgitation can cause relevant symptoms1,2.
In developed countries, tricuspid regurgitation (TR) is the most common pathology while tricuspid stenosis is extremely rare. TR can be of primary or secondary origin3. Secondary TR or functional TR (FTR) is the most frequent cause of tricuspid insufficiency4. The underlying cause of primary TR is a leaflet pathology or some congenital disorders such as Ebstein’s disease. FTR may be caused by annular dilatation as well as right ventricular enlargement and dysfunction as a consequence of left-sided heart disease from valvular or myocardial causes, right heart infarction or right ventricular pressure and volume overload (caused by pulmonary hypertension, cardiomyopathies and of other origin)1,3,5. Long-standing persistent or permanent atrial fibrillation or other arrhythmias may lead to the development of FTR by its effect on tricuspid annular dilatation1,5.
Surgical treatment of FTR in the context of left-sided valvular disease remains a matter of controversy with regard to accurate diagnosis, indication for surgery, surgical techniques and the late outcomes of surgical interventions4.
In the age of percutaneous valve interventions another treatment option for tricuspid valve dysfunction is evolving. Percutaneous tricuspid valve treatment is at an early stage and will be discussed elsewhere6.
This article focuses on indications, surgical techniques and results of tricuspid valve interventions.
Indications
All recommendations on the management of valvular heart disease in the 2012 joint ESC/EACTS guidelines concerning the indication for the surgical treatment of tricuspid valve disease are based on expert opinion only3.
In symptomatic patients with isolated severe tricuspid valve stenosis (TS), surgery is indicated (Class I). Regardless of the presence of symptoms, tricuspid surgery is indicated in patients with severe TS undergoing left-sided valve interventions. Since tricuspid stenosis is rarely observed in developed countries in contrast to developing countries, this publication will focus on the treatment of tricuspid regurgitation3.
With regard to the surgical indications for the treatment of TR, different indications for isolated tricuspid procedures and concomitant tricuspid procedures in the context of the surgical correction of left-sided valve pathologies exist.
In symptomatic patients with severe isolated primary TR without severe right ventricular dysfunction, surgery is indicated (Class I recommendation). Surgery should be considered in asymptomatic or mildly symptomatic patients with severe isolated primary TR as well as deterioration of right ventricular function or progressive right ventricular dilatation (Class IIa recommendation). After left-sided valve surgery, isolated tricuspid valve surgery should be considered in patients with severe TR, who are either symptomatic or who show progressive right ventricular dilatation/dysfunction, in the absence of left-sided valve dysfunction, severe right or left ventricular dysfunction, or severe pulmonary vascular disease3.
Concomitant to left-sided valve surgery tricuspid valve surgery is indicated in the case of patients with severe primary or secondary TR (Class I recommendation). Surgery should be considered either in the case of moderate primary TR or in patients with mild to moderate TR with dilated annulus (≥40 mm or >21 mm/m2) in the case of left-sided valve operations (Class IIa recommendation)3.
Surgical techniques
GENERAL CONSIDERATIONS
Isolated tricuspid surgery can be performed on the beating heart. The patient is put on cardiopulmonary bypass with cannulas in the superior and inferior caval vein. After snaring of the cava, the right atrium can be opened; coronary venous drainage is controlled by placing a vent in the coronary sinus. Both tricuspid repair and replacement can also be performed by a minimally invasive technique through a 5 cm right lateral thoracotomy.
Tricuspid valve repair
Several techniques for a tailored surgical treatment of the tricuspid valve exist. Surgical techniques can be divided into different categories (Table 1) depending on whether the level of the repair involves the annulus (annuloplasty) or the leaflets, or involves other techniques. If repair is impossible or the result of a repair effort is not satisfactory, tricuspid valve replacement can be performed.
ANNULOPLASTY TECHNIQUES
The “classic” De Vega annuloplasty consists of a plication of the posterior and anterior portion of the annulus with a double continuous suture, preserving the septal portion of the annulus. This technique was developed in the early 1970s and is one of the most frequently used techniques for surgical correction of FTR4. Several modifications were proposed by, for example, Antunes and Girdwood, Sarray and Duarte7,8.
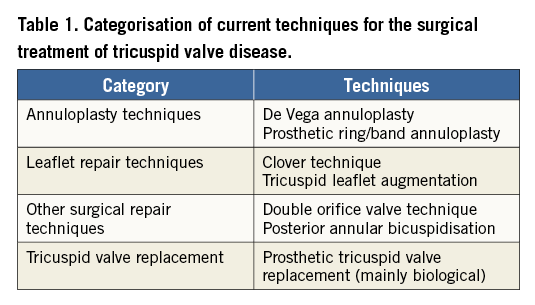
At present, the most frequently performed surgical procedure for repairing FTR secondary to tricuspid annular dilatation is undersized prosthetic tricuspid annuloplasty with devices such as flexible bands, rigid or semirigid annuloplasty rings (Figure 1). The current generation of tricuspid annuloplasty rings features a 3D geometry that respects the non-planar geometry of the native tricuspid annulus. As opposed to mitral rings, tricuspid rings are not closed and preserve the native annulus at the level of the triangle of Koch to avoid AV block. The undersized prosthetic tricuspid annuloplasty technique remodels the annulus, increases leaflet coaptation and reduces recurrent annular dilatation4.
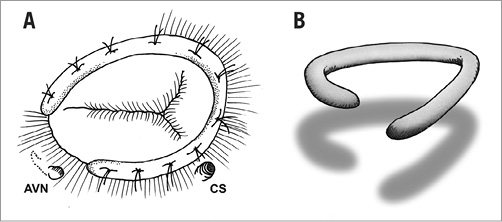
Figure 1. Prosthetic tricuspid ring annuloplasty is at present the most frequently performed surgical procedure for repairing functional tricuspid regurgitation. The ring is open at the level of the triangle of Koch to avoid impact on the AV node (A). The current generation of tricuspid annuloplasty rings features a 3D geometry that respects the non-planar geometry of the native tricuspid annulus (B). AVN: atrioventricular node; CS: coronary sinus
LEAFLET REPAIR TECHNIQUES
The “clover technique” was described by De Bonis et al and is similar to the Alfieri repair technique for mitral valve repair. The central parts of the free edges of the tricuspid leaflets are sutured together producing a clover-shaped valve; it is always combined with a prosthetic ring annuloplasty. The objective of this approach is to improve the efficacy of tricuspid valve repair in the setting of complex degenerative or post-traumatic pathologies4,9.
In case of leaflet tethering in addition to tricuspid annular dilatation, the leaflet augmentation technique described by Dreyfus et al increases the surface of leaflet coaptation by threefold and brings the coaptation zone down into the right ventricle at the level of the tethered posterior and septal leaflets, while maintaining leaflet mobility. To achieve this, the anterior leaflet is first detached from the anteroseptal to the anteroposterior commissure and then enlarged by the use of an autologous pericardial patch4,5,10,11 (Figure 2).
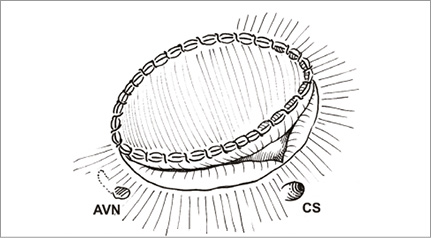
Figure 2. Leaflet augmentation technique: the anterior leaflet is first detached from the anteroseptal to the anteroposterior commissure and then enlarged by the use of an autologous pericardial patch.
OTHER SURGICAL TECHNIQUES
The rationale for posterior annular suture bicuspidisation is the finding that the primary anatomic problem in FTR is dilatation of the posterior tricuspid annulus12. The suture bicuspidisation is performed by placing a double pledget-supported mattress suture from the anteroposterior to the anteroseptal commissure along the posterior annulus (Figure 3)13.
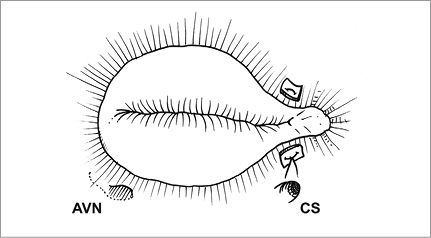
Figure 3. The posterior annular bicuspidisation is performed by placing a double pledget-supported mattress suture from the anteroposterior to the anteroseptal commissure along the posterior annulus.
The double orifice valve technique is performed by passing two pledget-supported mattress sutures from the middle of the anterior annulus to the septal annulus, aiming at a point located at two thirds of the length of the septal annulus measured from the anteroseptal commissure to avoid injury to the bundle of His (Figure 4).
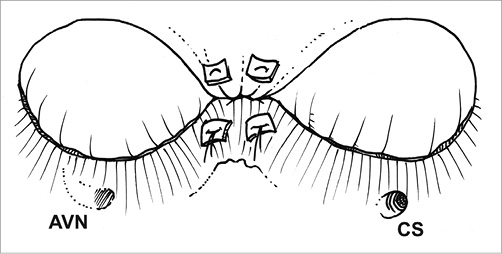
Figure 4. The double orifice valve technique is performed by passing two pledget-supported mattress sutures from the middle of the anterior annulus to the septal annulus.
TRICUSPID VALVE REPLACEMENT
If valve repair is impossible or if the result of a repair attempt is not satisfactory, tricuspid valve replacement needs to be carried out. In more advanced forms of tethering and right ventricular dilatation, valve replacement should be primarily considered3. Currently, the use of large-sized bioprostheses is favoured over valve replacement with mechanical valves3,14.
Results of surgical techniques & discussion
In the past, FTR was often underestimated and undertreated for two reasons. First, it was thought that the surgical correction of left-sided valvular heart disease would improve FTR sufficiently by reducing pulmonary hypertension and volume overload. Secondly, there was uncertainty about the optimal tricuspid valve repair technique, and a substantial rate of repair failure was reported15. It is now evident that left-sided valvular operations do not necessarily lead to an improvement of FTR and, more importantly, that residual TR is a prognostic factor for poor long-term outcome. Residual TR after mitral valve surgery is associated with an approximately 50% decrease in five-year survival. Furthermore, it was found that persistent TR is an independent predictor of development of heart failure with a hazard ratio of 2.4615-17. Dreyfus et al were able to show that concomitant tricuspid valve annuloplasty in mitral valve surgery based on tricuspid annular dilatation irrespective of the grade of regurgitation leads to a significant improvement in functional status and prevents the development of relevant regurgitation in the further course18.
Surgical treatment of FTR has mainly been focused on correction of annular dilatation over many years. Surgical strategies have changed within recent years with a more sophisticated approach to the treatment of FTR. Annuloplasty, however, still remains an important part of the surgical strategy.
A recent meta-analysis investigated the impact of ring versus suture (De Vega) annuloplasty on short-term outcomes, mortality and TR recurrence. Freedom from recurrent moderate TR at 15 years was significantly better in patients with ring annuloplasty (78.9±5.0% versus 50.5±5.9%; p=0.0107)19. Despite less recurrence of TR, 15-year survival did not differ significantly between the two groups (ring annuloplasty: 48.0±4.6% versus suture annuloplasty: 34.6±4.7%; p=0.441).
A recent retrospective study by Ren et al compared the results of De Vega annuloplasty with ring annuloplasty in 74 patients with concurrent left-sided valve operations. Complications and in-hospital mortality (2.9% De Vega versus 2.5% ring annuloplasty) were similar in the two groups (p=0.6755). Freedom from recurrent TR was significantly better in the ring annuloplasty group at 1 month, 6 months, 1 year, 2 years (log rank p=0.0377)20.
Tricuspid valve surgery can be performed either on the beating heart or with cardioplegic cardiac arrest. Besides reducing the time of myocardial ischaemia, another advantage of the beating heart approach is the possibility to assess the occurrence of atrioventricular block during the procedure. Baraki et al retrospectively compared the outcomes of 92 patients who underwent isolated tricuspid valve operations in either a beating or an arrested heart approach. Perioperative outcomes and 30-day mortality did not differ between the groups. Freedom from reoperation at 10 years was significantly lower in the beating heart group (arrested heart: 95% versus beating heart: 70%; p=0.039).
Recurrent or residual TR is a common finding after isolated annuloplasty. McCarthy et al investigated the outcomes of 790 patients with tricuspid valve annuloplasty using different techniques. Relevant TR (grade 3+ and 4+) was present in 14% one week postoperatively21. Based on these findings, it is evident that relying merely on annuloplasty techniques for the correction of FTR may not be sufficient. Surgical treatment of FTR needs to be performed with a more sophisticated approach. Recent publications suggest that FTR should be classified into three different stages. The first stage is defined as missing or mild TR without annular dilatation (annular diameter <40 mm) and normal leaflet coaptation. The second stage is characterised by annular dilatation (annular diameter >40 mm) and impaired leaflet coaptation (only at the edge level), leading to mild or moderate regurgitation. The third stage of FTR shows severe TR, annular dilatation and impaired leaflet coaptation with leaflet tethering and a tenting height of at least 8 mm below the plane of the annulus4,5,11. Surgical therapy should be tailored to the stages of the disease. Stage 1 does not require surgical intervention. In stage 2, valve annuloplasty is recommended and is most likely sufficient to restore leaflet coaptation. In stage 3 FTR, annuloplasty should be performed and, in addition, augmentation of the anterior tricuspid leaflet is recommended to provide a sufficient coaptation surface to ensure good long-term outcomes and avoid recurrent TR5.
One important aspect of the surgical management of tricuspid valve disease is to address transtricuspid pacemaker or ICD leads. McCarthy was able to show that transtricuspid pacemaker leads are an independent risk factor for the late recurrence of TR. At five years after tricuspid valve annuloplasty, 42% of the patients with a permanent pacemaker showed relevant TR compared to 23% of the patients without a pacemaker21. This finding suggests that late repair failure may be reduced by removing transtricuspid leads and replacing them with epicardial leads at the time of tricuspid valve surgery1. This way of lead management should also be followed in case of biological tricuspid valve replacement.
Conclusion
Most tricuspid valve interventions are performed for the treatment of functional tricuspid valve regurgitation. Ring annuloplasty is a key aspect of current surgical techniques and its durability is superior to suture annuloplasty techniques, although this does not translate into a survival benefit. Functional tricuspid regurgitation can be characterised into three different stages, and the surgical management should be tailored to these stages. In case of significant leaflet tethering, augmentation of the anterior tricuspid leaflet should be performed in addition to a ring annuloplasty in order to reduce the rate of recurrent regurgitation.
Conflict of interest statement
The authors have no conflicts of interest to declare.

