
Abstract
Tricuspid regurgitation (TR) is a frequently occurring valvular disease in the elderly population, the aetiology is functional in the vast majority of cases, and this valvular disease has become increasingly recognised as an independent predictor of morbidity and mortality. Early diagnosis and mechanical correction of TR is essential in impacting on the natural history of this valvular condition, but this is complicated by the fact that the majority of patients are asymptomatic, despite having moderate-to-severe TR. Multi-modality imaging, in particular echocardiography, is paramount in determining the mechanism, severity, and potential treatment options of TR. Patients with symptomatic severe TR often have multiple comorbidities and present with advanced tricuspid valve and right ventricular remodelling, thus limiting the treatment and prognosis. Indeed, this is a very heterogeneous and complex group of patients, where choosing the correct treatment may be challenging especially as the majority of patients present late, when surgical intervention is often associated with significant periprocedural morbidity and mortality. This has resulted in the development of numerous percutaneous transcatheter repair and replacement devices to treat this large group of high surgical risk patients. To impact on the natural history of severe TR will require earlier diagnosis and referral for treatment, a better understanding of the different stages of disease and potential treatment options, proven safe and efficacious percutaneous options, and an evidence base for earlier surgical or percutaneous intervention of significant TR, irrespective of symptoms.
Abbreviations
ASE: American Society of Echocardiography
CMR: cardiac magnetic resonance
CT: computed tomography
EROA: effective regurgitant orifice area
FTR: functional TR
LV: left ventricle
LVEF: left ventricular ejection fraction
PISA: proximal isovelocity surface area
RA: right atrium
RV: right ventricle
TR: tricuspid regurgitation
TV: tricuspid valve
VCA: vena contracta area
3D: three-dimensional
Introduction
Tricuspid regurgitation (TR) is characterised by a pathological amount of blood regurgitating from the right ventricle (RV) into the right atrium (RA) during systole, eventually leading to excess mortality and cardiac morbidity. While trivial TR is present in about 70% of normal individuals, the Framingham Heart Study showed that the prevalence of clinically significant (i.e., moderate or severe) TR in the general population reached up to 5.6% of women and 1.5% of men older than 70 years1,2. Although minimal or trivial TR may be considered a normal variant in structurally normal valves, moderate-to-severe TR is pathological and is caused by annular dilatation and/or leaflet abnormalities. Today, around 8% of people worldwide (i.e., more than 600 million) are aged ≥65 years, but this percentage is expected to reach about 17% by the year 2050 (i.e., more than 1.5 billion)3. Thus, the already notable prevalence of significant TR will most likely increase dramatically in the near future2. As a result of its frequency and clinical relevance, the fortuitous or incidental detection of blood regurgitating into the RA cannot suffice to mitigate the important and ever increasing pathological burden of TR. On the contrary, a more proactive attitude is essential nowadays if we are to make an impact on the natural history of this valvular disease.
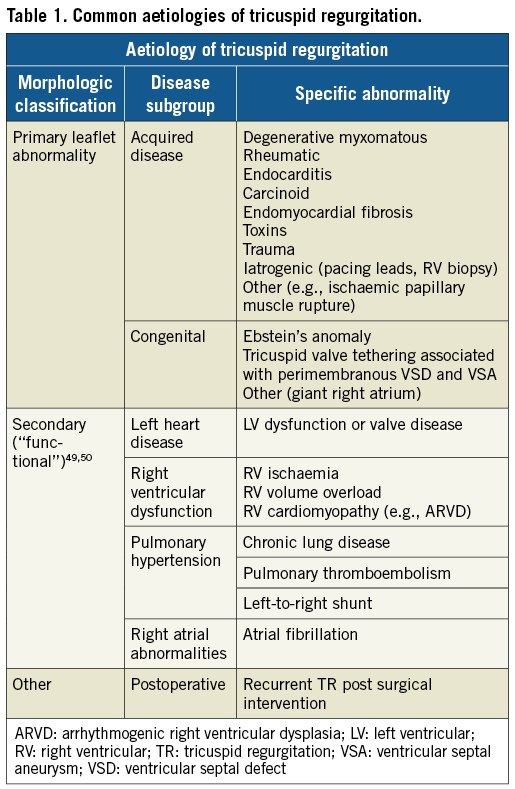
Cardiac imaging has to be considered for routine surveillance in selected groups of patients at high risk of developing TR (Table 1)4. Imaging is also fundamental to distinguish between organic (primary) and functional (secondary) TR. Organic TR is due to one or more anatomic abnormalities - either congenital or acquired - intrinsically affecting the tricuspid valve (TV) itself or the subvalvular apparatus. Functional TR (FTR) occurs in the presence of a macroscopically intact TV and subvalvular apparatus. FTR is due to any condition altering the geometrical relationships between the RV itself and the TV apparatus and/or enlarging and flattening the tricuspid annulus (Table 1). Recent epidemiological data indicate that FTR accounts for 90% of all-comers with severe TR, and in total 65% of FTR occurs in the context of pulmonary hypertension5. The pressure overload due to pulmonary hypertension that may or may not be associated with left-sided heart disease causes RV remodelling and tricuspid annular flattening and dilatation, eventually generating FTR. The annular dilatation usually occurs in a predictable direction towards the anteroposterior commissure (RV free wall), resulting in a more circular annular shape (the septal part of the annulus is part of the cardiac fibrous skeleton and thus relatively spared). TR begets TR by creating a vicious cycle of progressive RV dilatation, annular dilatation and leaflet tethering that results in increased TR severity and RV dilatation, eventually leading to RV dysfunction and failure6.
Cardiomyopathies with a dilated and hypokinetic phenotype can also produce per se an analogous remodelling process and eventually FTR. Intracardiac shunts, by causing RV volume overload, can represent a less common cause of FTR. Functional TR without pulmonary hypertension or any other identifiable cause is named by some authors “isolated” and in total accounts for 10% of all FTR diagnoses7. Whether isolated FTR should include patients in atrial fibrillation is debated.
In the present review, we discuss the clinical impact, diagnosis, prognosis and treatment of FTR, while at the same time proposing a practical diagnostic and management algorithm.
Clinical perspective
The pathophysiology and clinical manifestations of TR are characterised by the consequences of increased RA-RV pressure and volume and decrease in cardiac output. A major challenge in TR management is related to the fact that most patients remain asymptomatic for a protracted span of time, despite having moderate-to-severe TR. Moreover, when symptoms occur they can initially be insidious and difficult to diagnose or ascribe to the TV. The onset of fatigue, decreased exercise tolerance, peripheral oedema, hepatic congestion, decreased appetite, ascites/anasarca are non-specific and often erroneously considered non-TR-related. The presence of right heart failure symptoms and/or exertional dyspnoea in patients without left-sided heart disease should always raise the suspicion of severe TR. This is especially true in the elderly population where the subtle occurrence of symptoms makes the diagnosis and management particularly challenging. Physical examination can help attributing symptoms to the presence of severe TR, but jugular venous distension and a visible systolic V-wave indicating significant TR are present in only 35-75% of patients, and a murmur is present in less than 20% because of equalisation of pressures between the RA and RV4,8. The clinical presentation can also include symptoms mimicking left-sided heart disease because TR-induced RV volume overload impairs left ventricular (LV) filling by direct ventricular interaction through the interventricular septum. Having highlighted the intrinsic diagnostic difficulties of FTR, it is important that the cardiology community has a more proactive attitude towards this valvular condition.
Although there are studies attempting to evaluate the natural history and prognostic impact of FTR, their findings have sometimes been contradictory, with large variations in outcomes and debate as to whether TR severity, RV function, the underlying FTR aetiology or a combination are the predictors of poor outcome. This has been largely due to: a) the retrospective nature of these studies that are often limited by selection and ascertainment bias; b) the causes of FTR being diverse and often multiple; c) many of the conditions associated with or causing FTR being themselves markers of adverse outcome. This highlights the fact that the TR population is complex and heterogeneous, with a long-term prognosis that may be dependent on the associated comorbidities and/or specific aetiology, and where it may be challenging to determine the individual contribution of each of these factors on outcomes.
The most cited article demonstrating the negative prognostic impact of FTR showed that, in a retrospective evaluation of 5,223 patients, mortality increased with increasing severity of TR9. The one-year survival was 91.7% with no TR, 90.3% with mild TR, 78.9% with moderate TR, and 63.9% with severe TR. Moderate or greater TR was associated with increased mortality regardless of pulmonary artery pressure, and left ventricular ejection fraction (LVEF). Even after adjustment for age, LVEF, inferior vena cava size, and RV size and function, survival was worse for patients with moderate and severe TR compared to those without TR. More recently, moderate-to-severe isolated FTR was shown to be independently associated with excess mortality in FTR patients with preserved LVEF and pulmonary hypertension (either pre-capillary or post-capillary)10. Overall survival at one and four years in these patients (mean age 80 years) was about 60% and 20%, respectively10. The development of significant TR after left-sided valve surgery is not uncommon and is associated with an unfavourable outcome11. While preoperative pulmonary artery pressure can be similar in patients who will and will not develop FTR late after surgery, preoperative atrial fibrillation has been shown to be an independent risk factor for late post-surgical FTR11. Estimated survival free from cardiovascular death or reoperation in 335 patients (mean age 45 years) operated for left-sided valvular disease was 86% after 15 years in those who did not develop late FTR and 62% in those who did develop late postoperative FTR11. In a series of patients with cardiomyopathy and advanced heart failure referred for heart transplantation, the presence of FTR predicted RV dilatation, RV dysfunction and unfavourable outcome12. The one-year estimated survival, free from death or end-stage heart failure, in this group of patients referred for heart transplantation and any degree of FTR (mean age 49 years) was about 30%12. Cardiac implantable electronic devices have increased the quality and length of life for millions of patients. Positioning of an RV endocardial lead results in an increased risk of developing moderate/severe TR13. In those patients, distinguishing between organic TR (due to structural consequences deriving from lead placement or manipulation) and FTR due to the lead interference with leaflet mobility and coaptation (impingement) is challenging, if not impossible. In patients with implantable defibrillators or permanent pacemakers, significant TR has been found to be associated with excess risk of hospitalisation for heart failure and mortality14. The estimated one- and four-year survival in patients (mean age 80 years) with leads and significant TR has been reported to be approaching about 75 and 70%, respectively14.
Natural history studies focusing on isolated FTR are of clinical interest to distinguish between the prognostic role of TR per se vs. comorbidities associated with TR which may impair outcomes (e.g., pulmonary hypertension or left-heart diseases). In a study of 353 patients (mean age 70 years) with isolated TR, Topilsky and colleagues showed that severe isolated TR was present in 21.5% of patients and was associated with excess cardiac morbidity and mortality7. Notably, freedom from cardiac events was lower in isolated FTR with an effective regurgitant orifice area (EROA) ≥40 mm2, independent of all characteristics, right ventricular size or function, comorbidity or pulmonary pressures7. Overall, the estimated survival in isolated FTR patients is better than in all-comers with FTR; an EROA ≥40 mm2 approached 95% and 70% at one and four years, respectively7.
Diagnosis
In both the American15 and European16 guidelines for the management of valvular heart disease, the treatment algorithm for TR must begin with the assessment of the aetiology of regurgitation since the treatment of primary valvular disorders differs from the management of secondary TR. Once determined, the mechanism of regurgitation is defined. In the setting of FTR, the relative contributions of annular dilatation, RV dilatation/dysfunction with leaflet tethering, and pulmonary hypertension must be assessed. The severity of TR will also determine the recommendation for intervention, as will the consequences of regurgitation on cardiac remodelling, haemodynamics and end-organ function. The diagnostic test of choice is echocardiography; however, there are roles for both cardiac magnetic resonance imaging and computed tomography.
ECHOCARDIOGRAPHY
Echocardiography is instrumental in determining the aetiology of TR, evaluating the mechanism and haemodynamic consequences of TR on cardiac remodelling. Grading of the severity of the TR has been well described in the American Society of Echocardiography (ASE) guidelines17 as well as in the European Association of Echocardiography guidelines18. The ASE parameters most commonly used for this assessment and the algorithm for use of these parameters to guide the grading of severity are shown in Figure 1.
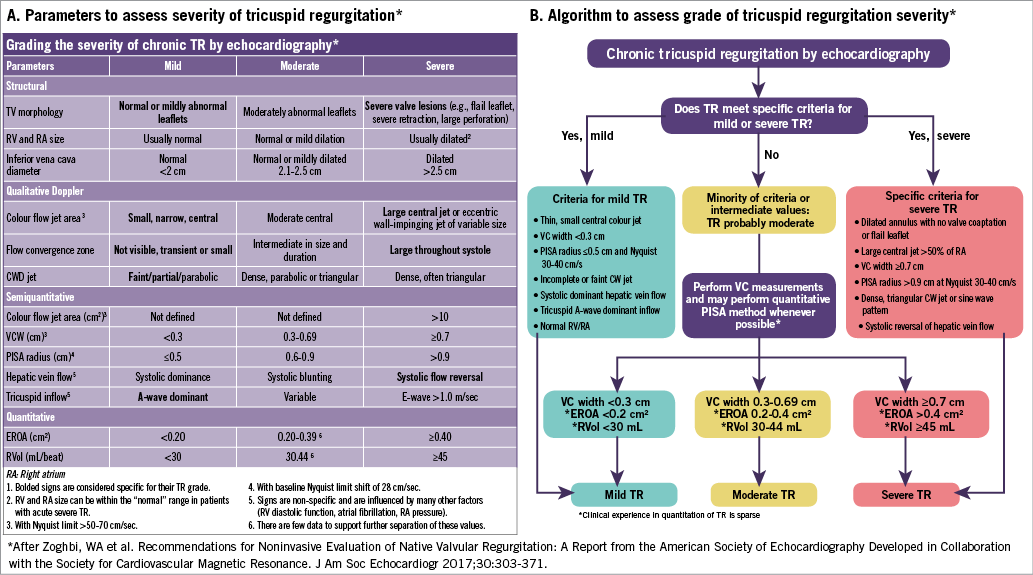
Figure 1. Parameters to assess the severity of tricuspid regurgitation according to the American Society of Echocardiography Guidelines17. A) Parameters to assess the severity of tricuspid regurgitation. B) Algorithm to assess the grade of tricuspid regurgitation severity.
To assess the severity of TR, imaging is used first to evaluate the structural abnormalities associated with TR: TV morphology, right heart size and function, and inferior vena caval size. The specific morphologic surrogates of severe TR include a flail leaflet and marked tethering with a large coaptation gap. The right ventricle and atrium are typically dilated except when regurgitation is acute. The inferior vena cava is dilated (>2.5 cm); however, this is a very non-specific sign.
Three-dimensional (3D) echocardiography has become an essential tool for the assessment of both right heart and TV morphology. Not only can it help to define the TV anatomy and the mechanism of TR19, but it has also been used to measure the size/geometry of the tricuspid annulus for quantification of regurgitation20 as well as pre-intervention planning21,22. 3D echocardiography is also the primary imaging modality for intraprocedural guidance of transcatheter interventional procedures20,23.
Qualitative assessment by Doppler includes a quick assessment of the size and direction of the colour jet, the presence and duration of a proximal flow convergence, and the density and shape of the continuous wave Doppler flow profile. If the jet is both central in origin and direction, and a large flow convergence zone is seen throughout systole, qualitative colour Doppler has greater specificity for severe TR. The parameters specific for mild TR include a small, narrow central colour Doppler jet, absent or very small flow convergence, and a faint or partial continuous wave spectral profile.
Semi-quantitative Doppler parameters indicating severe TR include the measurement of jet area >10 cm2, vena contracta width ≥7 mm, proximal isovelocity surface area (PISA) of >0.9 cm when the aliasing velocity is set to 28 cm/s, and an E velocity of >1.0 m/s. None of these parameters has high specificity. Only the systolic reversal of hepatic vein flow is considered specific for severe TR but should be evaluated in the absence of arrhythmias. A-wave dominance on pulsed wave Doppler interrogation is more specific for mild TR; however, it can only be assessed in normal sinus rhythm17.
Finally, the quantitative assessment of TR severity in the current guidelines relies on the PISA method. This method is simple and easy to perform24, and an EROA of ≥0.40 cm2 and regurgitant volume ≥45 ml qualify as severe. However, the PISA method uses a number of assumptions: 1) all flow that passes through the flow convergence zone also passes through the regurgitant orifice; 2) there is symmetrical, non-constrained acceleration of flow from all angles into an infinitesimally small, round, single regurgitant orifice producing a hemispheric geometry of flow. Although the first assumption is typically true, multiple parts of the second assumption are not true with PISA imaged using colour Doppler. First, what echocardiographic PISA images is Doppler velocity (not the speed of the blood), which is dependent on the angle of flow25. Second, with an orifice of finite size as occurs in the clinical situation, there is progressive flattening of the isovelocity surfaces as they approach the regurgitant orifice26. In addition, the shape of the tricuspid regurgitant orifice is often elliptical27 or stellate, which may result in significant underestimation of the regurgitant orifice by this method.
A number of studies have used 3D colour Doppler imaging to measure the vena contracta area (VCA) directly27-30. Using the validated measure of regurgitant jet area/right atrial area >34%31 and regurgitant jet area >10 cm2 to define severe TR32, a 3D TTE planimetered VCA of >0.75 cm2 was the most sensitive cut-off (sensitivity 85.2%, specificity 82.1%). More recently, 3D PISA has been used to quantify TR30. This method uses a vendor-specific software package to analyse the largest convergence zone, and a specific software which measures the 3D PISA. Few studies have used quantitation of TR by relative stroke volumes24,33,34. In these studies, a single-plane tricuspid annular diameter was measured from the four-chamber view and the tricuspid annular area calculated using a circular formula to calculate the tricuspid annular area. The sample volume for measuring the velocity-time integral was placed at the tips of the leaflets, unlike the recommended position of the sample volume for mitral quantitation, which is at the level of the annulus.
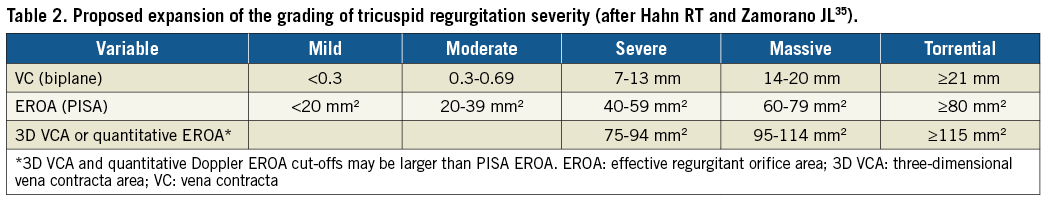
The current grading scheme for TR fails to capture the severity of regurgitation in patients currently presenting for treatment in early feasibility trials of transcatheter devices18,35. Although the European echocardiographic guidelines suggest that a grade of “massive” exists beyond “severe”, a new proposal for formal extension of the severity grades to “massive” and “torrential” has been suggested (Table 2). Importantly, this new grading scheme has not been critically assessed or correlated with outcomes. Before widespread adoption, this grading scheme will require further study.
COMPUTED TOMOGRAPHY (CT)
Although the use of CT for assessment of TR severity has not been well established, there is significant utility for this imaging modality, particularly in the era of transcatheter solutions for this disease. CT can be used for quantitative assessment of annular size, mechanisms and severity of malcoaptation, haemodynamic consequences for right atrial and right ventricular morphology and function, vena caval size and the course of the right coronary artery36-38. In patients with TR ≥3+, multidetector computed tomography (MDCT) demonstrated larger tricuspid annulus and RV dimensions and pronounced tethering of the anterior and septal tricuspid leaflet. The anteroposterior annulus diameter was independently correlated with the grade of functional TR37. A less favourable course of the right coronary artery (≤2.0 mm distance to the annulus) for current transcatheter annuloplasty techniques was observed at the level of the anterior tricuspid and posterior leaflets in 12.5% and 27.5% of patients with TR ≥3+, respectively38. In addition, CT measurements of TV morphology have been used to predict recurrence of disease following surgical repair. Tethering angles and height showed stronger correlation with preoperative TR severity, and multivariate analysis revealed that tethering height was an independent risk factor of postoperative recurrent TR (p=0.0069)39. Although direct measurement of the anatomic regurgitant orifice area has been used for mitral and aortic regurgitation, there have been no published studies performing this measurement for TR. A limitation of this method for TR evaluation is the difficulty in assessing the tips of a complex, non-planar orifice in the setting of thin, frequently poorly defined leaflets.
CARDIAC MAGNETIC RESONANCE (CMR)
CMR imaging for the assessment of TR severity has not been as extensively validated. Current CMR methodology40 requires the measurement of RV stroke volume (total stroke volume) by measurement of sequential short-axis images using the method of discs. Pulmonary flow using velocity-encoded phase-contrast images (forward stroke volume) is then subtracted from total stroke volume to quantify regurgitant volume. Pitfalls to the routine use of CMR for TR quantification include: 1) the need to acquire specialised CMR sequences; 2) measurement of total stroke volume relying on tracing endocardial boundaries at different phases of the cardiac cycle in multiple slices, where endocardial visualisation may not be optimal and the contribution of the outflow tract to total stroke volume remains controversial; 3) the prevalence of at least some pulmonic regurgitation being high in the normal population41 and possibly more significant in the TR population. Measuring the size and signal intensity of the cross-sectional jet area in the short-axis view on cine CMR may be another method to assess TR severity42.
Pharmacological and non-pharmacological treatment options for FTR
Treatment of FTR should follow an appropriate diagnostic process. The decision on whom, how and when to treat should be based on a comprehensive understanding of the mechanism of FTR, its severity, the characteristics of the right ventricle, the pulmonary circulation, the loading conditions, and the presence/absence of rhythm disturbances. Moreover, all of the diagnostic elements above have to be analysed in the context of the specific patient’s characteristics, comorbidities and surgical risk. Due to the lack of a solid scientific background, serial echocardiographic assessment of the RV function and remodelling, cardiopulmonary exercise test, magnetic resonance imaging, and right heart catheterisation may be used to integrate clinical findings and specific patient characteristics aiming at achieving an individualised decision making.
Diuretics can improve or delay the onset of symptoms, but they do not have an established role in preventing or delaying FTR progression. Similarly, whether a more aggressive strategy of rhythm control may indeed prevent TR progression needs to be established. Besides treating the underlying disease (if any), the only proven therapy for FTR is mechanical correction.
Surgical treatment of FTR
Surgery is the most established therapy for FTR. Available guidelines are mainly focused on FTR management at the time of surgery for left-sided valvular lesions15. The decision on whether to associate surgery for FTR in this group of patients is currently driven by the degree of FTR, the presence of significant annular dilatation, and/or right heart failure16. Late onset of TR is frequent in patients who have undergone surgery for left-sided valvular lesions, and reoperation for FTR is characterised by high surgical risk. Conversely, adding a tricuspid repair at the time of surgery for left-sided valvular lesions does not significantly increase the operative risk and may facilitate RV reverse remodelling and improve functional capacity43. Concerning surgery for isolated severe FTR, the recently delivered ESC guidelines recommend surgery (preferably repair) in patients either severely symptomatic or showing progressive RV dilatation/dysfunction (providing there is absence of severe RV/LV dysfunction and severe pulmonary hypertension)16. The current gold standard for surgical repair is ring annuloplasty with an incomplete semi-rigid prosthetic ring but some centres still perform the modified Kay bicuspidisation when the annulus is not severely dilated (i.e., <45 mm).
A number of recent studies have provided insights into the trends and outcomes of TV surgery:
– An analysis of 54,375 patients from the STS database undergoing TV surgery between 2000 and 2010 showed that TV repair was performed in 89% (annuloplasty only in 75.5% of repairs) concomitantly with another major procedure in the majority (86%)44. During this 10-year period, operative mortality decreased from 10.6% to 8.2%.
– A more recent, 10-year analysis of 5,005 isolated TV surgeries in the National Inpatient Sample files, representing 20% of hospital admission in the USA showed that operations per year increased from 290 in 2004 to 780 in 201345. In-hospital mortality was 8.8% and remained consistent over the study period. Interestingly, TV replacement was performed in 59.2% (bioprosthesis in 60.7%, mechanical in 39.3%) and repair in 40.8% with different operative mortality rates: mechanical valve 13.6%, bioprosthetic valve 9.1%, and repair 5.9%.
– A similar 10-year analysis utilising a US Nationwide Inpatient Sample of 9,194 patients undergoing TV surgery confirmed that isolated TV surgery is uncommon (15%) and that in most patients the TV is treated during concomitant cardiac surgery (85%). Patients undergoing isolated TV surgery are older with a higher risk profile and suffered high rates of postoperative morbidities as well as protracted hospitalisations. In-hospital mortality after isolated TV surgery is high (10.9% with replacement vs. 8.1% with repair) and has not changed significantly during the last decade. Also, isolated TV replacement was associated with much higher rates of permanent pacemaker implantation (34.1% vs. 10.9%).
The high mortality rates associated with TV surgery highlight the biggest challenge in treating this group of patients, i.e., the negative impact of late presentation on outcomes. Furthermore, it is unclear if the higher mortality rates with TV replacement compared to repair seen in the above studies are due to a selection bias since patients with more advanced TV and RV remodelling are more likely to be treated with valve replacement or due to the deleterious effects of a prosthesis on RV function or prosthetic complications.
Percutaneous treatment of FTR
As mentioned above, only a small fraction of patients are currently being offered surgical intervention, mostly due to high operative risk related to multiple comorbidities and late presentation or referral for intervention. This has sparked the development of numerous percutaneous transcatheter devices (Figure 2)6,46. These transcatheter devices have been developed either specifically for the TV or as mitral devices that are now being applied to the TV. In general, the majority of the tricuspid devices have been developed with the aim of reproducing surgical techniques and can be broadly divided into the following categories:
a. Direct suture annuloplasty: Trialign™ (Mitralign Inc., Tewksbury, MA, USA), MIA™ (Micro Interventional Devices Inc., Newtown, PA, USA), pledget-assisted suture tricuspid valve annuloplasty (PASTA).
b. Direct ring annuloplasty: Cardioband (Edwards Lifesciences, Irvine, CA, USA), IRIS (Millipede Inc., Santa Rosa, CA, USA), DaVingi (Cardiac Implants Ltd, Israel).
c. Coaptation enhancement: edge-to-edge with MitraClip® (Abbott Vascular, Santa Clara, CA, USA) or PASCAL (Edwards Lifesciences), TriCinch™ (4Tech Cardio Ltd., Galway, Ireland), FORMA (Edwards Lifesciences).
d. Valve replacement:
– Orthotopic: NaviGate (NaviGate Cardiac Structures, Inc., Lake Forest, CA, USA), TriSol (Trisol Medical, Haifa, Israel), Lux (Ningbo Jenscare Biotechnology Co., Ltd., Ningbo, China), TRiCares (TRiCares SAS, Paris, France).
– Heterotopic: TricValve® (P&F Products & Features GmbH, Vienna, Austria), Tricento® (NVT GmbH, Hechingen, Germany and NVT AG, Muri, Switzerland).
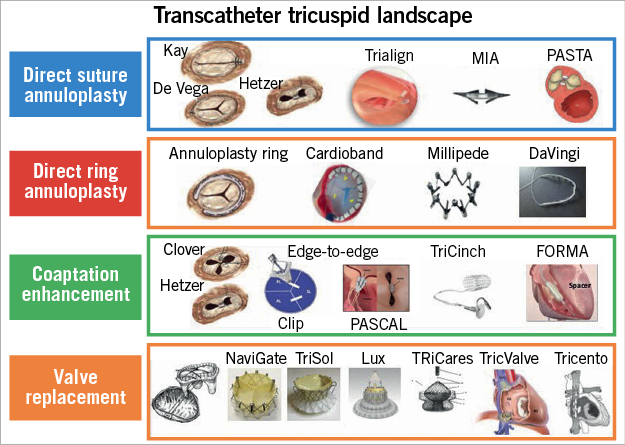
Figure 2. Percutaneous transcatheter devices for TR that are under development or investigation according to their main mechanism of action.
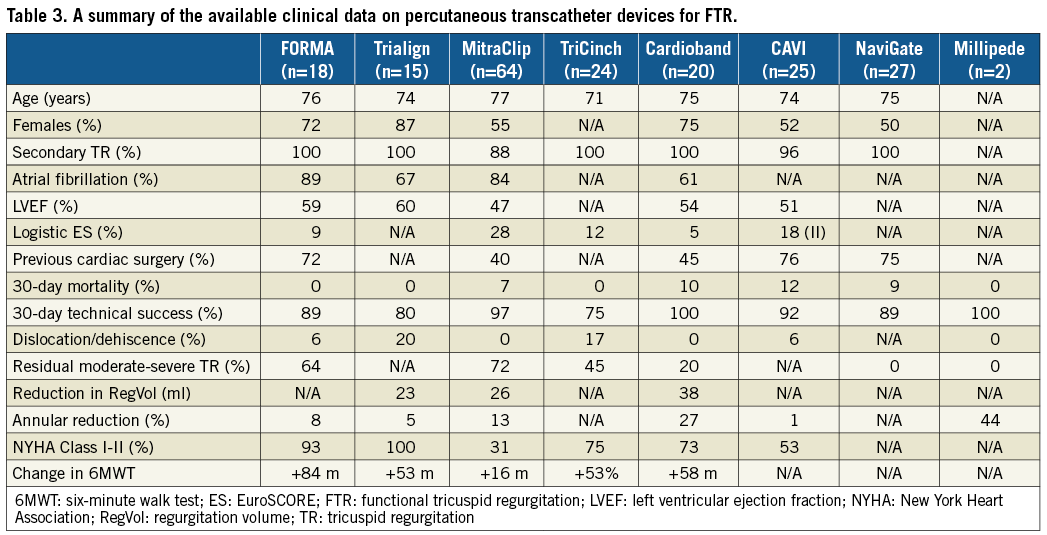
Table 3 summarises the preliminary data available for the devices implanted in humans under compassionate use or in early feasibility studies (EFS). We would like to highlight the following important observations regarding these data:
– Clinical data on many of the devices are still insufficient, thus limiting any conclusions regarding safety, efficacy and durability.
– All the studies have different primary endpoints and diverse measures for evaluating efficacy such as annular reduction, TR grade, vena contracta, PISA or effective regurgitant orifice area.
– The reduction in TR with most devices is modest, yet patients had clinically significant improvements in symptoms and quality of life.
Furthermore, the following caveats should be considered when evaluating the clinical data on these transcatheter devices as they may explain why the efficacy (as regards TR reduction) of some of these devices may be significantly different from their surgical counterparts:
– The majority of patients being enrolled in EFS are probably not the ideal patients for these devices as they have advanced TV remodelling that requires treatment with either multiple devices (i.e., annuloplasty plus edge-to-edge) or TV replacement.
– These are not the patients undergoing isolated TV surgery but are the ones who have been evaluated as inoperable by surgeons.
– The majority of the surgical data relates to patients undergoing FTR treatment at the same time as left-sided surgery.
– The annuloplasty devices that have moderate efficacy in the patients currently being treated will probably be much more effective in patients treated earlier or in patients similar to those undergoing surgical annuloplasty.
Conclusions
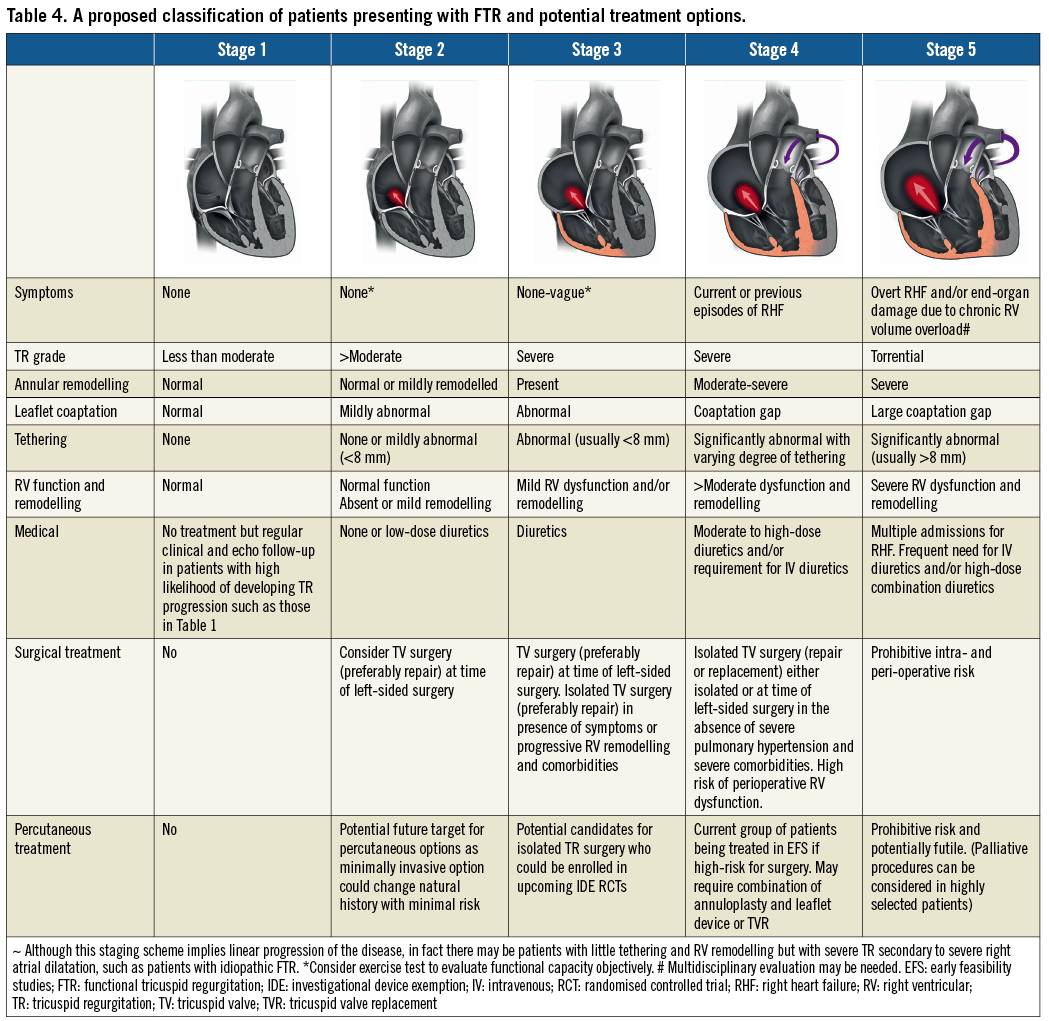
An important concept that needs to become widely accepted is that, when the annulus becomes dilated (planar and circular), it does not change after treatment of the primary cause of pressure or volume overload, thus implying that annular dilatation might be irreversible47. This would explain the rationale for annuloplasty in patients undergoing mitral surgery even in the absence of severe TR and for consideration of transcatheter therapies in the future for annular dilatation in asymptomatic patients with moderate-severe TR. One of the biggest challenges in selecting the appropriate treatment strategy is the fact that patients requiring isolated TV intervention are a complex group with multiple comorbidities presenting at different stages of TV disease. However, most patients currently being referred are often in quite advanced stages of TV and RV remodelling, which explains why surgical intervention may be associated with significant morbidity and mortality, whereas the efficacy of safer percutaneous interventions may be limited. This results in a very heterogeneous group of patients where choosing the correct treatment may be challenging. In Table 4 we provide a proposed classification to understand better the spectrum of patients presenting with FTR and the proposed treatment strategies. This classification expands on the previous three-stage classification of Dreyfus et al48 that may have been adequate for patients evaluated for surgical intervention but is not sufficient to describe the wide heterogeneity and complexity of the contemporary group of patients that we are currently evaluating and treating with transcatheter interventions.
If we are truly to make an impact on the natural history of TR and reduce periprocedural morbidity and mortality rates of TV intervention, it will require:
A better understanding of TV disease as well as the predictors and timing of progression.
Clinical and echocardiographic screening in certain high-risk groups of patients in order to recommend treatment before advanced TV and RV remodelling occurs, e.g., patients who undergo mitral valve intervention without TV intervention, long-standing chronic AF with atrial dilatation.
That surgical repair of tricuspid annular dilatation at the time of left-sided heart surgery should be standard practice.
Increased patient and physician awareness to promote earlier referral of symptomatic patients for TV intervention, which should be tailored according to the underlying pathophysiological mechanism of TR.
Proven safe and efficacious percutaneous therapies for patients considered high-risk for surgical intervention.
An evidence base for earlier surgical or percutaneous intervention of significant TR irrespective of symptoms.
Conflict of interest statement
A. Latib is a consultant for Medtronic, Abbott Vascular and Mitralign. R. Hahn is the Principal Investigator of the SCOUT trial for which she receives no compensation, and is a speaker for Abbott Vascular, GE Medical and St. Jude Medical. F. Grigioni received payments as a board member from LivaNova and grant funding from 4Tech.

