
Background
The current COVID-19 pandemic caused by SARS-CoV-2 began in November 2019 in Wuhan, China1, and has caused a global healthcare crisis with more than twenty million confirmed cases and almost 800,000 deaths as of 16 August 2020. The resulting burden on medical systems and the imposed social distancing measures in countries around the world have disrupted economies, healthcare systems, and ongoing clinical trials. Challenges have arisen due to quarantines and SARS-CoV-2 infections of hospital personnel and subjects, in-person follow-up appointment cancellations with a rapid shift to telemedicine, travel restrictions, supply chain disruptions of investigational products, cancellation of non-urgent procedures, and prioritisation of treating the surge in COVID-19 patients2,3.
In light of these changes, the US Food and Drug Administration (FDA), National Institutes of Health (NIH), and the European Medicines Agency (EMA) have released guidance on the conduct and management of clinical trials during this pandemic2,3,4. These recommendations emphasise the paramount importance of protecting the immediate safety of trial subjects and healthcare personnel while maintaining trial data integrity – particularly with respect to changes in clinical follow-up visits and other conduct that affect the investigational plan of ongoing studies. However, these guidelines do not completely address the longer-term consequences for cardiovascular clinical trials that will arise as a result of this global pandemic. We therefore seek to address more broadly the potential impact of an increase in cardiovascular adverse events due to COVID-19 and the concurrent changes in assessment and care-seeking behaviours that may lead to event underreporting. Both of these effects will probably have lasting implications for the management, conduct, analysis, and interpretation of trials in cardiovascular medicine (Figure 1).
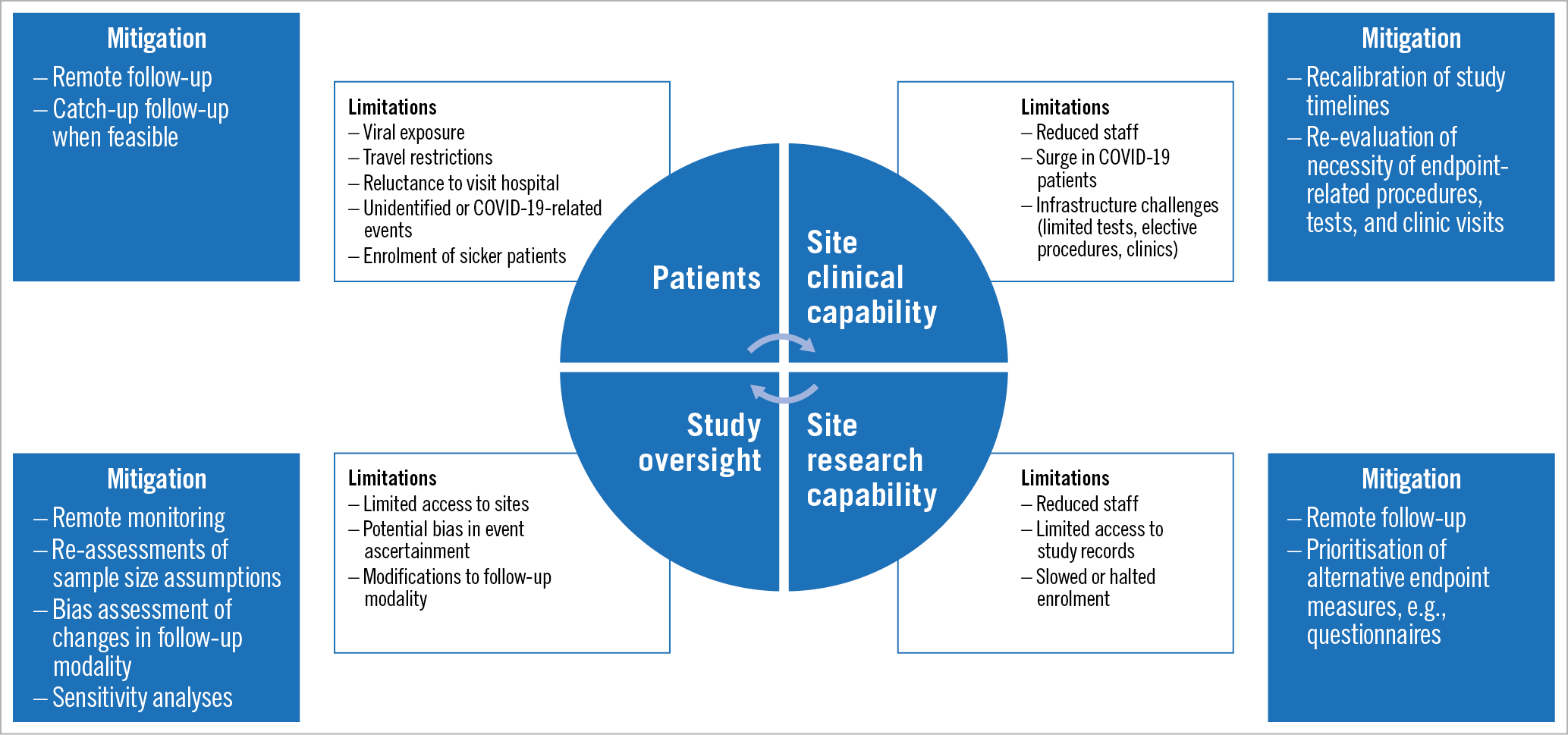
Figure 1. Impact of COVID-19 pandemic on cardiovascular clinical trials and proposed mitigation measures.
Impact of COVID-19 on cardiovascular outcomes
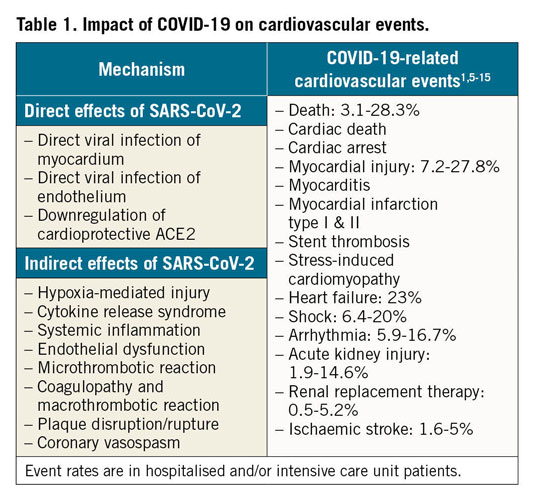
SARS-CoV-2 infection increases the risk of various cardiovascular events including death, thrombotic complications, shock, and arrhythmias (Table 1),1,5,6,7,8,9,10,11,12,13,14,15. Patients with cardiovascular disease (CVD) are particularly susceptible to severe complications related to SARS-CoV-2 infection, with an up to fivefold increased risk of mortality5,8,16. This is the result of both direct and indirect effects of SARS-CoV-2 infection on the cardiovascular system. For example, direct viral invasion of the myocardium and resulting myocarditis has been described post mortem, although the exact incidence and long-term effects remain unknown17,18,19. The indirect consequences of generalised hypoxia and COVID-19-induced cytokine release syndrome (CRS) may result in systemic inflammation and end-organ injury that can exacerbate and contribute to worse CVD outcomes5. Microthrombotic reactions or acute plaque disruption caused by a combination of endothelial dysfunction due to the generalised inflammatory response of CRS, direct viral invasion of endothelium, and the coagulopathy observed in hospitalised COVID-19 patients probably contribute to myocardial injury and other CVD-related complications6,7,20,21,22. These thrombotic complications in COVID-19 patients, including anecdotal reports of stent thromboses23, and the mortality benefit seen in anticoagulated patients with severe COVID-1924 will probably confound clinical trial results.
Other potentially relevant indirect effects are factors that correlate with CVD such as age, medication usage, and immune deficiency25. For example, the age-related increase in expression of angiotensin-converting enzyme 2 (ACE2), the host receptor for SARS-CoV-2 cell entry, in patients with hypertension and CVD may increase susceptibility to acquiring the virus, whereas, after infection, SARS-CoV-2 can downregulate ACE2 and block its multiple cardioprotective targets, worsening long-term outcomes26. It is also unclear whether SARS-CoV-2 directly or indirectly affects haemodynamics, in particular blood pressure and heart rate which are endpoints frequently collected in cardiovascular trials. The longer-term effects of infection on the cardiovascular system remain poorly defined.
Confounding and ascertainment considerations in clinical trials
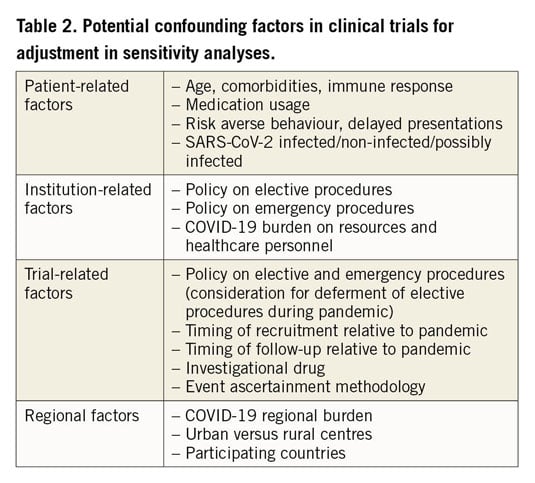
Confounders related to the pandemic will probably have a direct impact on the outcomes of ongoing clinical trials and should be considered in an individualised manner depending on the specific clinical trial context (Table 2). There are growing concerns about the accurate diagnosis and adjudication of clinical events in patients with COVID-19. For instance, determining whether myocardial injury is from viral myocarditis or CRS, spontaneous plaque rupture myocardial infarction (type I MI), or myocardial supply-demand mismatch (type II MI) has been a challenge in the context of the pandemic, particularly when imaging is not routinely obtained. Moreover, multiple reports of ST-elevation observed in COVID-19 patients with no evidence of coronary artery disease (CAD) on angiography have emerged, presumed to be SARS-CoV-2 myocarditis or sepsis-induced cardiomyopathy18,19,21,27,28.
Traditional patient and physician behaviour has also changed, and a new challenge has arisen in identifying and selecting patients needing revascularisation. With concerns about viral spread, more centres are turning to thrombolysis rather than transfers or direct primary angioplasty, and coronary imaging is being deferred27,29,30. At the same time, centres around the world are reporting a significant drop of acute ST-elevation myocardial infarction (STEMI) and non-STEMI patients presenting to medical centres and an up to 40% reduction in catheterisation laboratory activations compared to the same months in 201931,32. In parallel, there has been an increase in patients presenting with late complications of MI33 and a substantial rise in out-of-hospital cardiac arrests34,35. This is probably related to a combination of factors including a true reduction in MI rates due to stay-at-home orders, patient reluctance to seek care leading to missed non-fatal MIs and unrecorded fatal MIs, deferrals of interventions in critically ill COVID-19 patients, and possible missed or inaccurate diagnoses due to insufficient healthcare resources. Similarly, the rates of ischaemic strokes and mechanical thrombectomies have also decreased in certain regions36.
Other trial-specific therapies including drugs, interventions or devices may affect a subject’s risk of acquiring COVID-19 or their response. Several pathways are plausible: increasing high risk exposure if one arm of the trial requires more in-hospital care, mitigating risk factors in one arm due to better blood pressure or diabetes control, risk reduction through treating underlying CAD or structural heart disease (SHD), or direct effects of study drugs on the life cycle of SARS-CoV-2 or a host’s responses to it. Numerous medications commonly used in cardiovascular patients may interact with medications being used to treat COVID-1925. More directly, the anti-inflammatory and cardioprotective effects of cardiac medications including statins and ACEi/ARBs26,37, and the antithrombotic effects of antiplatelets and anticoagulants24 may directly affect outcomes of COVID-19 patients and can have an impact on study results if they are used differentially in clinical trials. Reports of theoretical safety issues associated with ACEi/ARB treatment in patients with COVID-19 have caused significant uncertainties among patients and physicians26. These have been addressed by various cardiovascular societies38,39. Because of these speculations, adherence to cardiovascular medications may have decreased or drugs may have been replaced during the pandemic, which could have an impact on prognosis in patients with CVD. We propose methods that can be considered in order to mitigate the effect of these factors on the integrity of cardiovascular trials.
Recommendations to mitigate impact on clinical trial data integrity
The imperatives during this pandemic have been to minimise exposure to and transmission of the virus, preserve limited healthcare resources, and prioritise treatment of COVID-19 patients during the surge of cases. It is in the context of this new benefit-risk paradigm that ongoing cardiovascular clinical trials be re-evaluated to address the impact on conduct, endpoint ascertainment methods, and outcomes. These considerations may lead clinical investigators, working closely with regulators and sponsors, to modify protocols when needed to protect patients and study personnel while optimising trial data interpretability and generalisability.
ONGOING ENROLMENT IN CLINICAL TRIALS
Aside from the priority placed on COVID-19-related clinical research, enrolment in most cardiovascular trials has been suspended unless the investigational treatment is deemed essential for emergent or urgent cardiovascular indications or compassionate use for a patient’s stability or survival. Trials evaluating patients with elective or semi-urgent indications have been paused. For example, active enrolment of patients in cardiogenic shock or receiving transplants may take precedence if the investigational treatments have promise of providing a clear benefit over standard of care40. Emerging clinical practice recommendations from the ACC/SCAI and clinical centres have been highly selective in identifying candidates for acute interventional treatment27,30,41,42. Additionally, a recent multi-society document has given recommendations for the safe reintroduction of cardiovascular services during the pandemic43. These same guiding principles are helpful to justify changes to enrolling patients in clinical trials. In some cases, trial interventions can be safely conducted and monitored in the subject’s home, and continued enrolment may be appropriate after considering the potential benefit to trial participants, the regional burden of COVID-19 on the healthcare system, and the risk to the patients and healthcare personnel.
FOLLOW-UP IN CLINICAL TRIALS
Clinical follow-up of ongoing cardiovascular trials has also adapted to the new imperative of safety first with imposed quarantines and site closures unavoidably resulting in mostly remote clinical follow-up. The FDA and EMA guidance documents provide general principles to maintain compliance and the integrity of ongoing clinical trials based on whether alternative follow-up methods can ensure patient safety. The FDA generally recommends that urgent or emergent changes to the protocol or informed consent can be implemented prior to independent review board (IRB) or FDA notification when the safety of patients is at stake and the change is not expected to have a significant impact on data integrity2.
Modifying effectiveness assessments also involves thoughtful consideration when methods of ascertainment are remote. Patients with the appropriate equipment can often measure their own vital signs, and clinicians can assess functional limitations due to angina and New York Heart Association (NHYA) class and identify basic physical examination findings such as increased work of breathing or peripheral oedema by telehealth40. Validated phone surveys, such as angina questionnaires and quality-of-life assessments, can be conducted remotely, submitted electronically, or done by available smartphone applications44. An application for the six-minute walk test in the home can be considered for heart failure patients45. Similarly, smart watches can be used to identify some arrhythmias (i.e., atrial fibrillation) or to obtain a 3-lead electrocardiogram (ECG)46. The additional risks posed by endpoints requiring on-site measurement such as imaging and blood draws need to be carefully weighed against their possible benefit to trial participants. The greatest challenge is presented by trials requiring standardised blood pressure measurement (e.g., ambulatory blood pressure monitoring, unattended blood pressure measurements, etc.), biomarkers, laboratory samples, directly observed drug therapy, or imaging assessments for primary safety or efficacy evaluation. It is important to balance the risk of patient or staff exposure, continuing therapy without surveillance, or missing an effectiveness measure. It may be reasonable to consider different outcome measures in situations where personal interaction with patients is impossible or prohibitive. If that is not possible, halting the trial may be the only option.
DATA QUALITY AND MONITORING
The inability to perform onsite monitoring during the pandemic and the potential for missing data and loss to follow-up will affect study quality in several ways. Until stay-at-home orders are lifted, remote monitoring programmes can be implemented to identify new safety concerns and evaluate primary endpoints, reduce missing data, and limit protocol deviations in real time. In general, the FDA and EMA have recognised the current limitations and the necessity of remote data collection and monitoring methods by phone and video visits2,4. Aside from providing ongoing monitoring of patient safety, what constitutes acceptable monitoring for assurance of data quality and integrity in support of regulatory decisions is highly context dependent. Resolving the practical challenges of remote monitoring and the acceptability of remote-only monitoring will involve dialogue among clinical investigators, regulators, sponsors, and site personnel. As the pandemic subsides, new measures and follow-up such as additional imaging may be useful to validate outcomes measured remotely or to identify events that may have been missed47.
Recommendations for clinical event ascertainment and event adjudication
It is likely that during the pandemic, particularly during surges of COVID-19 hospitalisations, patients lost to follow-up may have had unrecognised events including death, MI, or stroke. Cases of MI and ischaemic stroke in affected regions have dropped significantly31,32,36. This is presumed to be related in part to patients’ fears of hospitalisation and exposure to SARS-CoV-2 at the height of the pandemic. Equally, event identification that requires onsite diagnostic testing (e.g., 12-lead ECG, imaging, or biomarkers) that is not performed due to remote follow-up may also reduce event ascertainment and reporting.
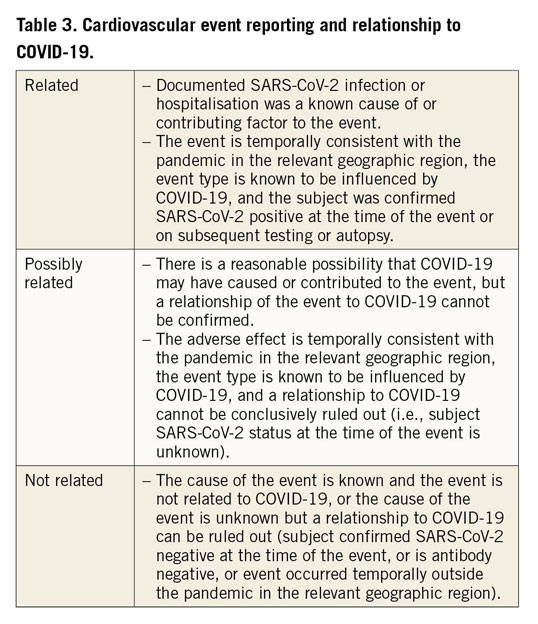
In contrast, we can expect a rise in COVID-19-related deaths, myocardial injury and thrombotic complications including stroke, venous thromboembolism, and possibly stent thromboses to have an effect on cardiovascular trials11,20. In this respect, careful event adjudication by independent clinical event committees (CECs) often results in updated charters and potential expansion of CECs to include members with COVID-19 expertise to enable re-adjudication of events starting from the onset of the pandemic in the relevant geographical locations. It will be important to classify events as related, possibly related or not related to SARS-CoV-2 infection (Table 3). While ultimately this may not have an impact on between-group comparisons in some randomised trials, a clear understanding of the underlying pathophysiology of events is essential to define the treatment effect of new therapies.
Recommendations for clinical trial analysis and interpretation
Statistical analysis plans (SAPs) may need to be modified based on various considerations including the increase in deaths of unknown cause (occurring at home or without autopsy), the increased loss to follow-up and missing data, asymmetric enrolment by clinical sites differentially impacted by COVID-19, and/or increased enrolment of emergent/urgent patients relative to stable patients. Careful consideration of alternative endpoint measurements and changes in the standard of care, which will affect baseline outcomes, may be appropriate. For example, more STEMI patients may be treated with thrombolysis without primary PCI, and fewer patients with angina or stent restenosis may receive invasive imaging. These changes in event rates may lead to updating trial sample sizes47. In addition, trial pauses will probably slow subject recruitment and enrolment, which can alter the distribution of events, leading to the need to revise power calculations.
Pre-specified additional sensitivity analyses in the SAP can enhance the scientific validity of trials in view of subsets of patients that may have different outcomes due to COVID-19-specific and/or healthcare system factors (e.g., SARS-CoV-2 positive/negative patients, pre-pandemic, intra-pandemic, and post-pandemic patients, differential methodologies for endpoint ascertainment, region of clinical site)40,47. Sensitivity analyses can help to identify potential interactions between COVID-19-related events and the trial intervention. It may be useful to plan a pre-/post-COVID-19 analysis for all trials that were recruiting at the start of the pandemic. FDA and EMA recommendations to provide patient-level listing of the impact of COVID-19 on follow-up modalities, missing data, and protocol deviations are critical but will need to be supplemented by study-specific analyses to guide interpretation of clinical trial results in the context of COVID-192,47.
Considerations for resuming clinical trial enrolment and restoring original plans for follow-up and monitoring should be outlined in protocol amendments to minimise disruption in the transition to a post-pandemic clinical research environment.
Economic implications
Just as the COVID-19 pandemic is having a profound effect on the general economy, the impact on the clinical research ecosystem is serious and will be long-lasting. Central to the impressive advances in cardiovascular medicine that have occurred over the past two decades is a highly evolved clinical research infrastructure driven by a community of professionals at contract research organisations (academic and for-profit), core laboratories, and clinical sites. Interruption of reliable funding required to sustain this infrastructure will probably be destabilising. Similarly, the economic impact of the COVID-19 pandemic on sponsors and biotech/medical device developers in the cardiovascular sector is profound. Particularly vulnerable are pre-revenue start-up companies, whose success and survival are often based on the ability to run efficient clinical trials that demonstrate the utility of their product. The unanticipated interruption and future slowdown of subject recruitment, along with the difficulties with subject follow-up, will increase the time and capital requirements to complete studies. To preserve resources, many companies have already resorted to furloughs, salary reductions, and lay-offs. As a consequence, companies may need to raise additional capital, which is particularly difficult in the current economic environment, with a concern that some companies with ongoing trials will be unable to raise sufficient capital to complete studies, let alone sustain development and innovation.
Conclusion
The COVID-19 pandemic has disrupted health care and medical research worldwide. A new paradigm focused on safety through distancing has changed the conduct of cardiovascular clinical research in profound ways. Direct effects of SARS-CoV-2 infection on the cardiovascular system can be expected to have an impact on patient outcomes in differing ways and result in adapting trial management, methodology, and outcome measures depending on the context of individual clinical trials. The economic impact of the COVID-19 pandemic will force clinical investigators, regulators and sponsors to be flexible, leverage new technology, and find new approaches to ensure that ongoing studies can be completed with scientific rigour within the confines of new social and economic challenges.
Conflict of interest statement
W. Wijns reports an institutional research grant and honoraria from MicroPort. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), and Deutsche Forschungsgemeinschaft (SFB TRR219) and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic and ReCor Medical. G.G. Stefanini has received research support (to the institution) from Boston Scientific, and speaker fees from B. Braun, Biosensors, and Boston Scientific. A. Kaplan receives grant support from Abbott Laboratories, Boston Scientific, Edwards Lifesciences and Medtronic, and is a founder and director of Conformal Medical, a venture-backed start-up developing left atrial appendage closure technology. The other authors have no conflicts of interest to declare.
Disclaimer: this article reflects the consensus views of the writing group and does not necessarily represent the practices, policies, requirements, or recommendations of the US Food and Drug Administration. Furthermore, the use of the word “required”, “must”, or “should” in the document is not intended to indicate a US Food and Drug Administration requirement.
Supplementary data
To read the full content of this article, please download the PDF.

