
CASE SUMMARY
BACKGROUND: A 59-year-old male who had received neoadjuvant chemotherapy for a bladder cancer 10 months previously was admitted for recurrent chest pain episodes after ureteral reimplantation.
INVESTIGATION: ECG, serum cardiac markers, serum haemoglobin, transthoracic echocardiography, coronary angiography, QCA, 3D-QCA, FFR for serial stenosis, endomyocardial biopsy.
DIAGNOSIS: Left ventricular systolic dysfunction, multivessel coronary heart disease.
MANAGEMENT: Blood transfusion, myocardial revascularisation.
KEYWORDS: anthracycline cardiotoxicity, coronary heart disease, fractional flow reserve, intermediate coronary lesions, left ventricular systolic dysfunction, quantitative coronary angiography, serial coronary stenosis
PRESENTATION OF THE CASE
We present the case of a 59-year-old male, smoker, with a diagnosis of locally advanced node-negative transitional cell bladder cancer (T2N1Mx). He was treated with neoadjuvant chemotherapy consisting of MVAC cycles (methotrexate, vinblastine, adriamycin and cisplatin) followed by radical cystectomy and a second chemotherapy cycle. Seven months later the patient developed an ureteric stricture with obstructive renal failure (serum creatinine 2.8 mg/dl). During admission, the patient suffered recurrent chest pain and three clustered episodes of ventricular fibrillation, successfully reverted with shocks of 200J. As compared with the basal ECG, ST-depression in II, III, aVF and in precordial leads V4-V6, as well as T-wave flattening in all member derivations and V4-V6 was observed during the pain episodes (Figure 1). CK rose to 367 μg/l (CK-MB 41 μg/l) and peak troponin I to 14.4 μg/l. Urgent coronary angiography was performed in his referring hospital and showed left dominance with moderate angiographic lesions in both the left anterior descending (LAD) and circumflex (LCX) coronary arteries. The left ventricular cavity was of normal size, with a left ventricular ejection fraction (LVEF) of 40% by visual estimation on echocardiography; no segmental wall motion abnormalities (WMA) were observed. The findings were attributed to an eventual cardiotoxic side effect of the anthracyclines administered around the cystectomy, and the patient was discharged after stabilisation with medical treatment.
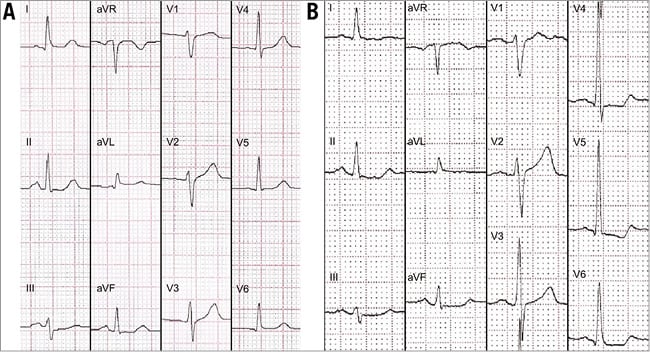
Figure 1. ECG changes during the episodes of chest pain. Basal ECG (A) in sinus rhythm, without repolarisation abnormalities. During the pain episodes (B), the ECG showed transient ST-depression in II, III, aVF, V4-V6, as well as T-wave flattening in all member derivations and V4-V6.
Laparoscopic ureteral reimplantation was scheduled three months later and was performed uneventfully. However, in the days following the intervention the chest pain episodes recurred, mimicking the same repolarisation abnormalities previously described in the ECG, but the markers of myocardial damage remained normal. Serum haemoglobin was 8.4 g/dl. On the echocardiogram, LVEF was 41% by biplane Simpson’s method, without clear regional WMA (Moving image 1-Moving image 3). The patient was transferred to our hospital, where a new coronary angiography was performed (Moving image 4-Moving image 8), showing moderately calcified coronary arteries, with moderate lesions in the proximal and distal LAD, as well as in the proximal LCX and second obtuse marginal (OM2) branch (Figure 2). Using bifurcation-dedicated quantitative coronary angiography (QCA) software (QAngio XA 7.3; Medis bv, Leiden, The Netherlands), the diameter stenosis (DS) of these lesions was 34%, 25%, 15% and 17%, respectively (Figure 3).
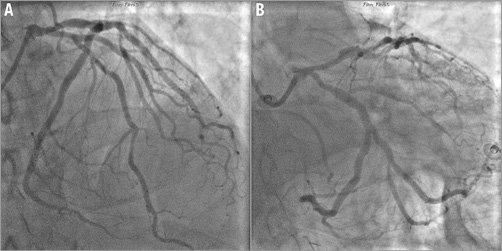
Figure 2. Coronary angiography. Cranial view showing the LAD (A) and caudal view showing the LCX (B). Serial moderate stenosis in the proximal LAD around the take-off of the first septal branch, distal LAD, proximal LCX and one of the branches of the second OM.
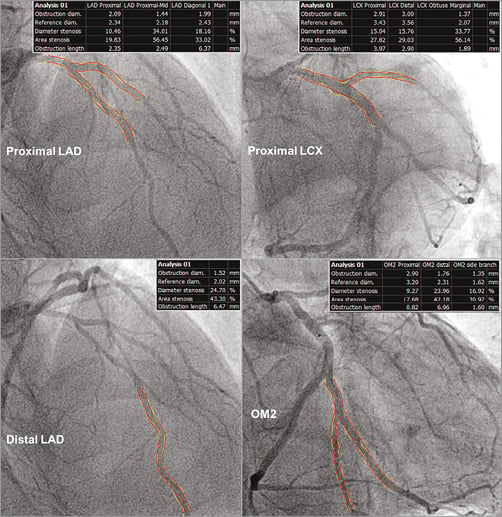
Figure 3. Bifurcation-dedicated quantitative coronary angiography (QCA). In all cases the maximal diameter stenosis was below 40%.
At this point, the dilemma arises as to which is the most reasonable direction to proceed in order to elucidate the cardiac problem of the patient and to guide its appropriate management. Should we concentrate on the coronary arteries? Is it time for an endomyocardial biopsy (EMB)? Perhaps just medical therapy?
How would I treat?
THE INVITED EXPERTS’ OPINION

The authors present a case of a patient with bladder cancer treated with an initial cycle of chemotherapy consisting of methotrexate, vinblastine, adriamycin and cisplatin (MVAC), followed by a radical cystectomy and a second cycle of MVAC. At a later stage, in conjunction with renal failure due to a ureteric stricture, the patient suffered chest pains, ventricular fibrillation and had a small elevation of cardiac enzymes. Left ventricular ejection fraction (LVEF) was 40% and an urgent coronary angiogram revealed atherosclerosis but no significant lesions. An ECG revealed clear changes at the time of chest pain compared to baseline. Then, when the patient returned for ureteral reimplantation three months later, postoperatively he again suffered chest pains and similar ECG changes, although without a rise in cardiac enzymes, and with no episodes of ventricular fibrillation. A new coronary angiogram was performed but still revealed no significant coronary lesions and the ejection fraction was 41%.
The authors do not report what the baseline LVEF was, but it has to be assumed that the anthracyclines and their cardiotoxic effect were the cause of the moderately reduced LVEF observed. The most disconcerting information, however, is the cluster of episodes of ventricular fibrillation (VF) and its root cause. The patient had experienced chest pains with ECG changes shortly before going into VF. Although there was a small increase of enzymes, this could also be explained by the periods of cardiac arrest during VF. In the patient’s next episode of chest pain, during which similar ECG changes were observed, there was no release of cardiac enzymes.
The most important issue is whether this 59-year-old patient will be at risk of future episodes of VF, and does the patient need an intracardiac defibrillator (ICD)? We also need to evaluate the patient for a possible treatable cause in the coronaries.
A left ventricular angiogram in conjunction with coronary angiography may have been helpful to rule out a variation of Takotsubo cardiomyopathy as a cause, since both episodes happened during times of stress and the diagnosis can sometimes be difficult with ultrasound alone. We would also schedule a cardiac magnetic resonance imaging exam for evaluation of viability of the myocardium and evaluation of a possible Takotsubo cause. Although the coronary arteries did not exhibit any significant lesions, this does not necessarily rule out the possibility of an occult coronary lesion. Therefore, we would at least perform a cardiac nuclear stress test to rule out the possibility of cardiac ischaemia or redo the angiography and perform fractional flow reserve testing in all the major epicardial arteries. It would also be reasonable to perform a cardiac biopsy but this may not provide any more answers.
Unless testing revealed significant coronary disease (which would be treated of course) or a certain Takotsubo cardiomyopathy, we would most likely treat the patient medically (with a beta-blocker and an ACE inhibitor) and implant an ICD.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

The 59-year-old patient with bladder cancer treated with surgery and chemotherapy experienced two episodes consistent with acute coronary syndrome (ACS), the first complicated with VF. Angiography showed moderate stenoses. The LV function was reduced (EF=40%).
The potential aetiologies include chemotherapy-related cardiotoxicity, coronary spasm or thrombotic occlusion with spontaneous recanalisation. Our thinking and our strategy would be as follows.
Anthracycline cardiotoxicity leads to a reduction in LVEF that becomes significant months or years after chemotherapy1. However, in rare cases, acute cardiac injury mimicking ACS occurs within a few days after anthracycline infusion. The seven-month period between chemotherapy and ACS symptoms makes a diagnosis of acute anthracycline toxicity doubtful. On the other hand, LV dysfunction may be related to anthracyclines and this would require treatment as covered in the guidelines2.
Vasospastic angina should be taken into consideration. This may manifest as the classic Prinzmetal angina with ST-segment elevation or as ST-segment depressions resulting from diffuse spasm of coronary segments. Non-specific ST changes may also result from anthracycline toxicity3,4. Moreover, there are case reports linking cisplatin therapy and the induction of vasospastic angina5. Therefore, we would try to confirm the coronary spasm6. The vasospastic attacks usually resolve themselves rapidly and are difficult to document angiographically. Therefore, we would perform a provocation test with intracoronary acetylcholine. If diffuse spasm was induced, we would start conservative treatment: smoking cessation, aspirin, statins and calcium channel blockers6. In case of focal persistent coronary spasm we would consider spot stenting. We would recommend 12 months of DAPT6. If the malignancy did not limit the patient’s life expectancy, we would consider ICD implantation.
According to the ESC guidelines, a myocardial biopsy should be considered in patients with suspected anthracycline cardiomyopathy7. However, in the current patient we would not perform it as this would not influence treatment.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The stenosis analysis with bifurcation-dedicated 3D-QCA (QAngio 3D XA; Medis bv, Leiden, The Netherlands) resulted in a DS of 41% in the proximal LAD, 38% in the distal LAD, 32% in the proximal LCX and 65% in the OM2 (Figure 4). Although neither 2D- nor 3D-QCA analysis provided conclusive results about the severity of the coronary lesions, the clinical course of the patient suggested ischaemic heart disease rather than anthracycline cardiotoxicity. Therefore, a functional assessment of the lesions’ severity by means of fractional flow reserve (FFR) was indicated. After infusion of intravenous adenosine, the FFR in the distal LAD was 0.67. The pressure wire was then manually pulled back to the segment between the proximal and distal lesions in the LAD, obtaining an FFR there of 0.73 (Figure 5A). Likewise, after maximal vasodilation, the FFR was 0.73 distally to the lesion in OM2 and 0.76 between the lesions in OM2 and the proximal LCX (Figure 5B). After correcting the FFR for the presence of serial stenosis8, we obtained the following estimated FFR values for each lesion: proximal LAD 0.71, distal LAD 0.91, proximal LCX 0.75, OM2 0.97, thus indicating that the truly flow-limiting stenoses were the proximal lesions of both the LAD and the LCX, instead of the distal ones, as the results of 2D- and 3D-QCA seemed to suggest.
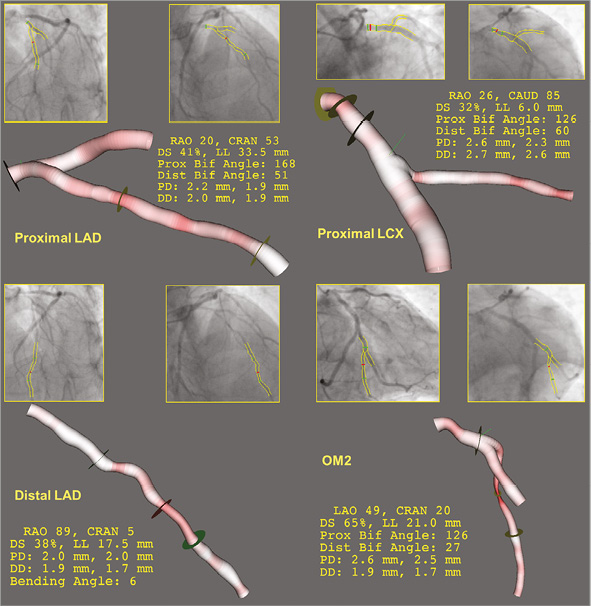
Figure 4. Bifurcation-dedicated 3D quantitative coronary angiography (QCA). Only the lesion in the small side branch of the OM2 is angiographically severe in 3D-QCA.
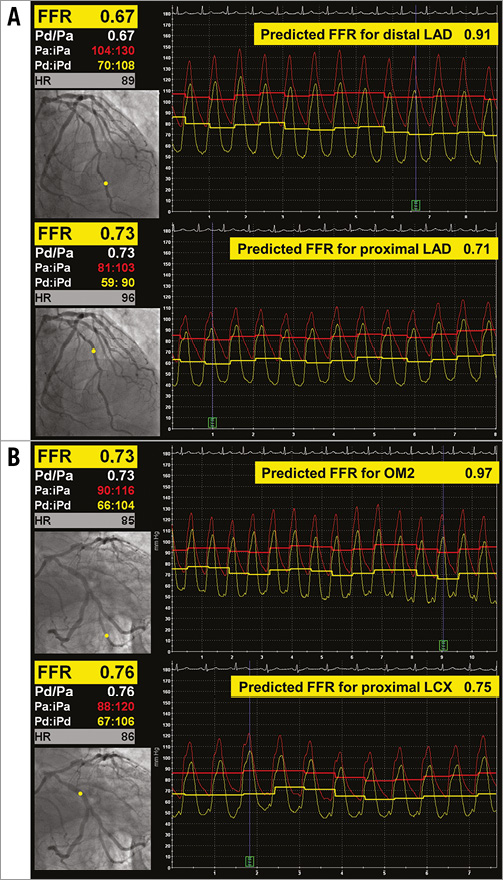
Figure 5. Functional assessment of severity. Fractional flow reserve in the serial lesions of the LAD (A) and of the LCX-OM2 (B). Pullback of the pressure wire, measuring distal to both lesions (Pd, upper panel) and between the lesions (Pm, lower panel). Measured FFR displayed on the left, predicted FFR for distal and proximal lesions displayed on the right.
The functional SYNTAX score9, including only those lesions with an FFR ≤0.80, was only 19, but a surgical revascularisation was preferred because of the recurrent vesical bleeding episodes and the eventual need for reintervention. Red cell concentrates were transfused and the patient stabilised under medical treatment. Bypass surgery with arterial grafts in Y, namely left internal mammary artery to the LAD and right internal mammary artery to the OM, was successfully performed with a single anastomosis in each case. The echocardiogram at discharge showed LVEF 70% (Moving image 9-Moving image 11).
Discussion
This case illustrates the limitations of angiography to evaluate the severity of coronary stenosis, due to foreshortening, vessel overlap, calcification or other factors10. Calcium deserves particular attention, because the edge-detection algorithms of most angiography systems are currently based on weighting the first and second derivatives of the density function, so the presence of calcium in the vessel wall, albeit in a moderate or mild amount, can significantly alter the edge detection, resulting in underestimation of the stenosis severity11. In this case, moderate calcification of the vessel could be clearly noted in the angiography and might explain, in part, the marked underestimation of the stenosis severity on angiography. Although the use of bifurcation-dedicated software and 3D-QCA has substantially improved the accuracy of conventional QCA12-14, the interference of calcium in the detection of the vessel edge is an inherent limitation to any angiography-based quantitative method.
The limitations of angiography must be especially borne in mind when a discrepancy between the clinical course and the angiographic severity is observed, such as in the current case. The antecedent of anthracyclines administration introduced some confusion in the interpretation of the findings, and was advocated to explain the malignant arrhythmias and the deterioration of the systolic function in the absence of angiographically severe coronary stenosis. Nonetheless, the cardiotoxicity of doxorubicin is normally dose-dependent, and the incidence after short MVAC cycles used, such as neoadjuvant therapy in bladder cancer, is minimal15-17. It is also uncommon for cardiac toxicity to appear months after discontinuation of the chemotherapy, instead of manifesting during its administration18, but that possibility cannot be totally ruled out. Conversely, several signs strongly suggested ischaemia: recurrence of chest pain coupled with reversible repolarisation changes in the ECG, elevation of cardiac markers under stress (obstructive renal failure), ventricular fibrillation without symptoms of heart failure. In case of such a clinical discrepancy, the functional assessment of the stenosis is fully justified. Patients with multivessel disease benefit from functional revascularisation guided by FFR as compared with solely angiographic guidance10,19. Nonetheless, FFR measurements in serial stenosis are still challenging, but the predicted FFR for each lesion can be reliably calculated8. The formulas used for the correction require the coronary wedge pressure (Pw) that can be measured only after balloon inflation, something hardly justifiable if no percutaneous coronary intervention is performed. Nonetheless, in most cases, the formulas render stable FFR values with negligible changes for a range of Pw between 0 and 30 mmHg, particularly if the distal lesion is not haemodynamically significant such as in this case; therefore, a fixed Pw (e.g., the end-diastolic pressure of the left ventricle) can be assumed. The predicted FFR indicated that both proximal lesions in LAD and LCX were haemodynamically severe, instead of the distal ones. This finding, however, at variance with QCA, fitted much better into the clinical picture and explained the globally depressed LVEF without WMA, as a case of LM equivalent in a left dominant anatomy.
The recommendations for an EMB are quite liberal and include suspected anthracycline cardiomyopathy as class IIa (level of evidence C), but the clinical presentation must be heart failure to set up a reasonable indication7. If that had been the case and the patient were under anthracycline therapy, EMB could be the most sensitive and specific method to monitor cardiac toxicity20,21. However, in the absence of symptoms of heart failure and with just an antecedent of chemotherapy 10 months ago, the diagnostic efficiency might be debatable.
Current guidelines give a class I recommendation for both surgical and percutaneous revascularisation in stable patients with multivessel disease if the SYNTAX score is ≤2219. The low functional SYNTAX score (=19) and the unstable presentation might favour percutaneous revascularisation. However, the patient’s symptoms are unlikely to correspond to an unstable plaque (essentially stable clinical condition until a concurring additional stress, such as the obstructive renal failure or the surgical intervention, destabilised it), and the risk of vesico-ureteral bleeding under dual antiplatelet therapy was not negligible. Therefore, after discussion in the Heart Team and with the treating urologists, a surgical revascularisation was proposed. The favourable clinical course and the normalisation of LVEF after a single anastomosis in each graft can be considered as an indirect sign that the predicted FFR for serial stenosis succeeded in identifying the truly flow-limiting lesions.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Echocardiogram after chest pain episodes. 4-chamber view.
Moving image 2. Echocardiogram after chest pain episodes. 2-chamber view.
Moving image 3. Echocardiogram after chest pain episodes. 3-chamber view.
Moving image 4. Coronary angiography. Non-dominant RCA.
Moving image 5. Coronary angiography. LAO caudal (spider) view.
Moving image 6. Coronary angiography. RAO caudal view.
Moving image 7. Coronary angiography. Caudal view.
Moving image 8. Coronary angiography. Cranial view.
Moving image 9. Echocardiogram at discharge, after surgical revascularisation. 4-chamber view.
Moving image 10. Echocardiogram at discharge, after surgical revascularisation. 2-chamber view.
Moving image 11. Echocardiogram at discharge, after surgical revascularisation. 3-chamber view.
Supplementary data
To read the full content of this article, please download the PDF.
Echocardiogram after chest pain episodes. 4-chamber view.
Echocardiogram at discharge, after surgical revascularisation. 2-chamber view.
Echocardiogram at discharge, after surgical revascularisation. 3-chamber view.
Echocardiogram after chest pain episodes. 2-chamber view.
Echocardiogram after chest pain episodes. 3-chamber view.
Coronary angiography. Non-dominant RCA.
Coronary angiography. LAO caudal (spider) view.
Coronary angiography. RAO caudal view.
Coronary angiography. Caudal view.
Coronary angiography. Cranial view.
Echocardiogram at discharge, after surgical revascularisation. 4-chamber view.

