CASE SUMMARY
BACKGROUND: A 42-year-old man with no cardiovascular risk factors or history was admitted for evaluation of an episode of transient ischaemic attack (TIA). He had no cardiovascular risk factors and his physical examination was unremarkable.
INVESTIGATION: Physical examination, electrography, transesophageal echocardiogram, coronary angiogram.
DIAGNOSIS: Intrapulmonary shunting, in the presence of arteriovenous malformations, possible hereditary haemorrhagic telangectasia
TREATMENT: Transcatheter occlusion of multiple pulmonary arteriovenous malformations using an AMPLATZER vascular plug.
KEYWORDS: pulmonary arteriovenous malformations, Rendu-Osler disease, transient ischaemic attack
PRESENTATION OF THE CASE
A 42-year-old man was admitted to the local Neurology Department to undergo evaluation of an episode of transient ischaemic attack (TIA). He had no cardiovascular risk factors and his physical examination was unremarkable. As a part of routine diagnostic procedures, he was referred to our echocardiography laboratory to undergo a transesophageal echocardiogram (TEE) for evaluation of potential cardio-embolic causes of the cerebrovascular disease and to investigate the presence of a patent foramen ovale (PFO). A TEE with saline contrast was performed, demonstrating the presence of a significant amount of bubbles in the left atrium (LA) consistent with the presence of a relevant right-to-left shunt despite no evidence of atrial or ventricular septal defects. The late timing of appearance of bubbles in the LA, after five heart cycles, was not compatible with inter-atrial septal defects such as a PFO but rather strongly suggestive of the presence of an intrapulmonary shunting and allowed us to differentiate intracardiac shunting from intrapulmonary shunting, as a result in this case of the presence of arteriovenous malformations. The patient was affected by nasal telangectasias, recurrent epistaxis and gastrointestinal bleeding, suggestive of hereditary haemorrhagic telangectasia (HHT).
The finding was subsequently confirmed as the patient, after informed consent was obtained, underwent a right heart catheterisation with pulmonary arteriography that demonstrated the presence of multiple pulmonary arteriovenous malformations (PAVMs). Figure1 shows the presence of a PAVM with a simple angio-architecture.
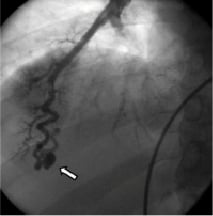
Figure 1. Selective pulmonary angiography demonstrating the presence of a pulmonary arteriovenous malformation (PAVM) with asimple angio-architecture. Arrow indicates the PAVM.
Given the likely causal link between the presence of the PAVMs and the embolic TIA, we decided to treat PAVMs. The case was discussed in our multidisciplinary team: several potential therapeutic options could be adopted: 1) transcatheter occlusion with AMPLATZER® Vascular Plug (AVP) (St. Jude - AGA Medical, Plymouth, MN, USA); 2) embolotherapy with coils; 3) embolotherapy with detachable balloons; 4) surgical treatment.
How would I treat?
The Invited expert’s opinion
Large pulmonary fistulae provide the third most common pathway for paradoxical embolism after the patent foramen ovale (PFO) and atrial septal defects. They are often associated with hereditary haemorrhagic telangiectasia as in the case presented. There may be multiple large communications1 or just one as in the case presented. Detection requires selective angiography or non-selective angiography with magnetic resonance or computed tomography. Suspicion may rarely arise from a plain chest x-ray1. Like the PFO, pulmonary artery fistulae have been associated with migraine headaches with or without aura2.
The presented case is particularly favourable for catheter-based closure of the fistula. The pulmonary artery at the entrance of the fistula beyond the last non-fistulating pulmonary artery branch (at about 8 o’clock in the picture) measures roughly 3-4 mm.
I suggest the following treatment:
The patient receives 5,000 units of heparin intravenously. The femoral vein is punctured in the groin without skin incision. A0.018 inch steerable guidewire is inserted and a 4Fr catheter is introduced over it. The catheter is advanced into the right pulmonary artery. A Y-connector allows dye injections to guide advancement of the steerable guidewire followed by the catheter into and perhaps through the fistula marked by the arrow. An AMPLATZER® Vascular Plug 4 (St. Jude-AGA Medical, Plymouth, MN, USA) measuring 6×7mm in diameter x length (Figure 2)3 is introduced into the distal pulmonary artery, right about where it turns horizontally at 8o’clock. A dye injection through the 4 Fr catheter is used to ascertain correct placement before the plug is unscrewed from the pusher cable. The plug will not be immediately totally occlusive, but experience shows that total occlusion of the fistula will occur over subsequent hours.

Figure 2. AMPLATZER Vascular Plug 4 (courtesy St. Jude-AGA).
An alternative would be the insertion of coils through the 4 Fr catheter. However, it can be assumed that more than one coil would be required and later recanalisation in spite of initial thrombotic occlusion is quite frequent and a well-known bane of coil occlusion. It has to be said, however, that even a recanalised segment containing coils would probably retain larger clots and hence prevent paradoxical embolism. At the end of the procedure, the patient puts his finger on the groin and walks out of the catheterisation laboratory, just like after donating blood.
Conflict of interest statement
Dr. Meier receives research funding and is part of the speaker bureau of AGA St. Jude-Medical.
How would I treat?
The Invited expert’s opinion
In this patient who was admitted to the hospital because of transient ischaemic attack, a consistent right-to-left shunt due to intrapulmonary shunt was demonstrated by the TEE bubble study; selective pulmonary angiography demonstrated a PAVM. Based upon the angiographic features of PAVM, we would proceed with coil embolisation of the fistula.1,2
Conflict of interest statement
The author has no conflict of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF CASE
Actually transcatheter embolotherapy is the recommended treatment for PAVMs in patient with HHT1. In this case, we decided to perform a transcatheter occlusion of PAVMs using an AMPLATZER Vascular Plug (AVP). Due to the peculiar anatomical features of the lesions and the technical complexity of the interventional procedure, we decided to adopt a therapeutic strategy, agreed with the patient, of staged procedures to occlude the remaining PAVMs to be performed weeks to months apart.
The procedure was performed via a right femoral venous approach. A 6mm AVP was delivered through the guide catheter to the target vessel and then released; a final angiogram performed after five minutes showed complete occlusion of the vessel (Figure3). In the same procedure, an additional 8mm AVP was successfully implanted with no appreciable residual flow at the angiography. The post-procedural clinical course was uneventful and the patient was shortly after discharged home. Thereafter, likely because of his clinical stability and the non-recurrence of TIA, the patient refused to complete the planned therapeutic schedule. However, after nearly three years of well-being, the patient abruptly developed dyspnoea due to development of a massive haemothorax involving the right lung as demonstrated by computed tomography (CT) (Figure4). Urgent drainage was performed with immediate symptomatic improvement and the patient was scheduled for a new pulmonary artery angiography. The angiography confirmed the presence of two PAVMs, one of which sub-pleural likely related to the occurrence of the haemothorax. Notably, the vessels occluded during the first procedure were not re-permeabilised. A percutaneous occlusion of the PAVM likely responsible for the haemothorax was performed using a 8mm AVP (Figure 5A and B, Moving image 1 and 2). A6mm AVP II device was implanted with no residual flow into the second PAVM (Figure 6A and B). In this case we decided to implant an AVP II: this device has a more densely woven nitylon wire and three occlusive segments. However the relatively long length of this device can preclude its use when the feeding artery is too short and several side branches are present.
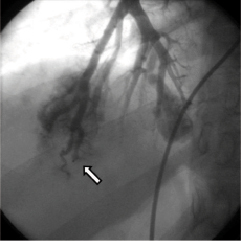
Figure 3. Final angiogram obtained five minutes after the deployment of the AMPLATZER Vascular Plug device showed total occlusion of the target vessel (arrow).
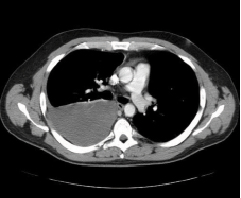
Figure 4. Massive haemothorax involving the right lung. The patient abruptly developed haemothorax three years after the first procedure.
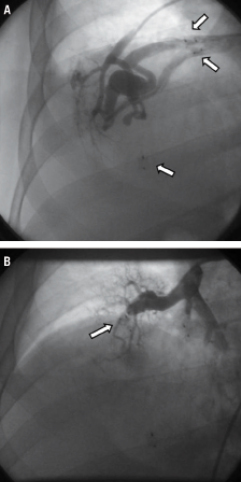
Figure 5. A) Pulmonary angiography performed four years after the first procedure showed the presence of a large PAVM with a more complex angio-architecture, likely responsible for the haemothorax. Arrows indicate devices previously implanted. B) Contrast injection performed at the end of the procedure demonstrated no residual flow after the deployment of the AMPLATZER Vascular Plug.
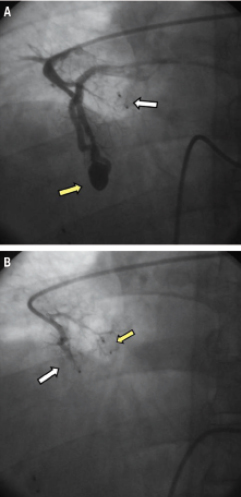
Figure 6. A) Angiography showed also the presence of a PAVM with a simple angio-architecture (yellow arrow). White arrow indicates the device previously implanted. B) Final angiogram demonstrating the complete occlusion of the PAVM. In this case we decided to perform the procedure using a AVP II (white arrow). It is possible to appreciate the long length of AVP II in comparison with AVP I (yellow arrow).
After discharge the patient was scheduled for regular radiological follow-up with magnetic resonance imaging (MRI). The latest MRI performed in November 2009 demonstrated the development of two new small PAVMs in the left upper lobe and no recanalisation of previously treated PAVMs 62 months after the first procedure and 23 months after the second procedure, respectively. He has not experienced recurrences of TIA or haemothorax up to the latest available follow-up.
Discussion
It is estimated that at least 80% of PAVMs occur in the context of HHT with the evidence that HHT-related PAVMs are more frequently multiple (35-65% of cases), bilateral and may more often lead to severe complications than solitary ones2,3. Surgical treatment, consisting of a lobectomy, is now rarely required and transcatheter closure has become the standard care for treatment of PAVMs, being a safe and far more conservative option4. Coils and detachable balloons have been used for occlusion of PAVMs, however risk of embolisation, potential for recanalisation and incomplete occlusion predisposing to stroke have been frequently reported as untoward sequelae4. To overcome the partial efficacy of earlier therapeutic options, alternative devices, such as AVP, have been developed. In our case, the occlusion of PAVMs using AVP devices was safe and effective regardless of the development of new multiple PAVMs three years after the procedure, which is typical of natural disease progression in HHT. Of note, our patient has not developed TIA or cerebrovascular complications.
PAVMs are associated with an increased risk of transient ischaemic cerebral events and ischaemic strokes. When occurring in the HHT patients population these events may have particularly devastating clinical consequences considering the young age at onset (41years of age in HHT compared to 63 years in the general population)5. Our case suggests that in patients with HHT associated with neurological complications, it may be crucial to assess the potential presence of a right-to-left shunting, possibly due not only to presence of PFO but also to PAVMs. Despite perfused PAVMs may be found in up to 23% of patients with HHT following occlusion by means of embolotherapy6, repermeabilisation of the treated vessels was not observed in our case after three years from the index procedure, suggestive of the effectiveness of percutaneous occlusion of PAVMs with the use of AVP and AVP II devices. This phenomenon is, however, related to the growth of non-occluded PAVMs and/or to the residual perfusion, more often than to recanalisation of the occluded PAVMs.
Three years after the first procedure, our patient abruptly developed a massive haemothorax involving the right lung and an urgent drainage has been needed. Our case demonstrated that a screening for PAVMs in patient with HHT should also be performed in consideration of the risk of life-threatening complications and the potential implications for treatment. The complication experienced is intrinsically related to the progressive nature of PAVMs which may manifest with growth of pre-existing lesions or development of new ones in different locations, as confirmed also by a scheduled follow-up MRI performed in November 2009. In the light of this peculiar feature of PAVMs along with the availability of effective therapeutic options to manage the most threatening lesions, it is mandatory for clinicians to provide an appropriate long-term follow-up for patients affected by HHT who have PAVMs, as recommended1, to detect disease progression and guide targeted interventions. Although still a matter of debate, multidetector thoracic CT with thin-section reconstruction is currently accepted as the best non-invasive imaging modality to evaluate potential perfusion of previously treated PAVMs or to detect development and/or growth of feeding vessels1,5. We did prefer MRI as a follow-up monitoring method in the reported case also in order to limit the patient’s radiation exposure.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Pulmonary angiography showing the presence of a PAVM with a complex angio-architecture and the devices previously implanted.
Moving image 2. Final result of the procedure demonstrating no residual flow after the deployment of the AVP device in the feeding vessel of the complex PAVM.

