Abstract
Pulmonary arteriovenous malformations (PAVM) describe a broad group of complex vascular malformations, often associated with multi-system diseases and with palliated complex congenital heart disease. They can have major clinical consequences, resulting in strokes, cerebral abscesses, cyanosis and, infrequently, rupture into the pleural space. The best approach to their investigation and interventional treatment is via a multidisciplinary pathway which should be focused in high-volume centres with on-site thoracic and cardiovascular surgical back-up. The availability of computed tomography (CT) and a broad cathlab inventory aid procedural planning and success. The results of interventional treatment are very encouraging and are applicable to an increasingly broad group of patients thanks to improvements in interventional techniques and a significant expansion of the inventory of vascular occlusion devices.
Introduction
The diagnosis and management of pulmonary arteriovenous malformations (PAVM) requires input from many disciplines and subspecialties. Haematology, hepatology, cardiology, respiratory medicine and radiology are usually involved with the initial diagnosis, as well as planning and managing interventions and coordinating ongoing care. PAVM are most accurately defined as low-resistance abnormal vessels which connect the pulmonary arterial and pulmonary venous systems, bypassing the normal lung microvasculature.
The association between PAVM and various syndromes and iatrogenic physiologies means that referral pathways, imaging modalities and treatment thresholds are diffuse1.
Aetiology and classification
PAVM are known to be heavily associated with hereditary haemorrhagic telangiectasia (HHT), with approximately 25% of those with a diagnosis of HHT having PAVM. Acquired conditions such as Fanconi syndrome, schistosomiasis and hepatic cirrhosis are associated with PAVM. There is also a strong association between the pathophysiological state following palliative surgery for congenital heart disease and PAVM. The exact cause of this last association is unknown but it probably relates to diversion of hepatic venous blood away from the pulmonary arteries, starving the lungs of an as yet unidentified “hepatic factor” which in normal circulation may suppress PAVM development2. PAVM are generally classified as macrovascular or microvascular, with microvascular PAVM being associated with the acquired forms. There is, however, crossover and variation within individual patients and across the congenital and acquired subtypes. Microvascular disease can be described on pulmonary angiography as a rapid-filling homogeneous “blushing” of the lung fields, representing a vast network of tiny abnormal vessels bypassing the capillary network where gas exchange takes place1,3.
Clinical presentation
Many PAVM remain asymptomatic but they tend to get bigger with the passage of time, and the effective right to left shunt can result in significant chronic hypoxia. Due to their bypassing the filtering mechanism of the lung bed, PAVM can also facilitate the passage of thrombus from the systemic venous to the systemic arterial circulation, leading to embolic strokes akin to those seen in patients with patent foramen ovale (PFO). The inherent abnormality and fragility of these vessels renders them susceptible to bleeding and on occasion rupture, leading to massive haemothorax (Figure 1)4.
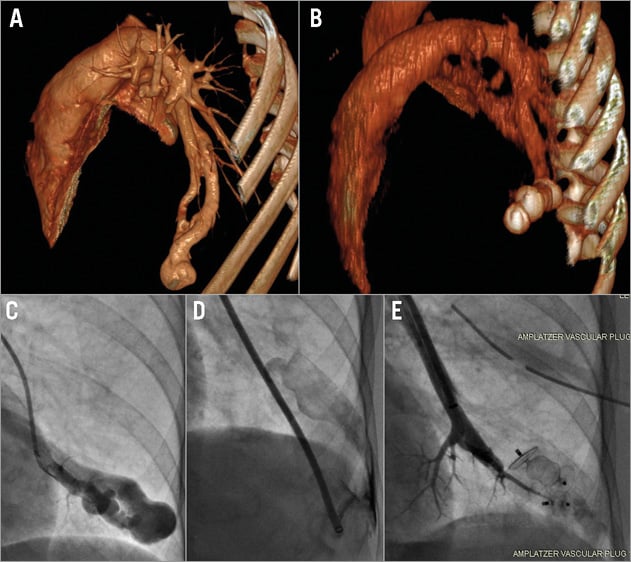
Figure 1. Emergency management of rupture during PAVM occlusion. A) A 3D computed tomography reconstruction of a large single LLL PAVM; an angiogram of the same lesion is seen in panel C. The lesion lies on the pleural surface of the lung. An injection during the procedure (D) shows a major complication as the delivery sheath has perforated the PAVM into the left pleural cavity causing a large haemothorax. In order to stem the bleeding, a transseptal puncture was quickly performed. The vein draining the PAVM was identified and both the venous (draining) end of the PAVM as well as the proximal (feeding) arterial supply were occluded with vascular plugs. Two vascular plugs can be seen in these positions in the acute follow-up CT scan (B) and the result can be seen angiographically in panel E.
Diagnosis and investigation
Diagnosis is usually by one of two routes.
1. Investigation of symptoms: low oxygen saturation, cryptogenic stroke, brain abscesses or spontaneous haemothoraces should prompt investigation for PAVM.
2. Investigation as part of work-up for associated conditions: patients with HHT will undergo screening for PAVM and regular surveillance for signs of the condition developing. A high index of suspicion in the other associated conditions usually leads to early diagnosis and management.
A bubble contrast echocardiogram can be diagnostic of the condition. The pattern of contrast return to the left atrium can usually differentiate between PAVM and a PFO. With PAVM the direction of contrast return is from the relevant pulmonary vein rather than from across the septum. There is also usually a relative delay in the contrast return with PAVM as it needs to pass through the right heart, the malformation and then the pulmonary vein before appearing in the left atrium. With a PFO the contrast moves almost immediately into the left atrium, and the dispersion of contrast originates from the atrial septum and the PFO itself. A positive bubble study with a bubble pattern suspicious for PAVM should be followed up with computed tomographic angiography. This will usually locate and define macrovascular PAVM and is often adequate to delineate microvascular disease as well. If there is any doubt, then direct pulmonary angiography by cardiac catheterisation is the gold standard for diagnosis and evaluation of the lesions2-4.
Medical management
Treatment of the underlying cause may prevent progression and may also decrease the size and effect of the PAVM. This is most obviously seen when treating patients with microvascular PAVM in hepatopulmonary syndromes and in those following a bidirectional cavopulmonary anastomosis (BCPS). The key disease drivers here are related to abnormal hepatic flow, resolved by liver function recovery or transplant in hepatorenal syndrome, and by completion of the Fontan circulation with restoration of the hepatic venous blood flowing through the pulmonary bed in those PAVM related to a BCPS2. Microvascular PAVM tend not to be amenable to direct interventional management and instead rely on these therapeutic options. In contrast, macrovascular varieties show minimal response to medical therapy and often require interventional treatment.
Interventional management
Identification of one or more significant macrovascular PAVM should lead to assessment of the balance of risks to that patient of intervention. The number, complexity and position of the defects, along with assessment of related and unrelated comorbidities should be balanced with the rate of progress of the PAVM and any consequences that have been suffered such as a brain abscess or stroke. It is not possible to prescribe absolute indications for interventional occlusion; instead, each patient should be considered on their individual merits.
The key imaging modality for planning interventional occlusion of a PAVM is a CT angiogram (Figure 1). This will allow planning of the required inventory as well as gauging the complexity and timing of the procedure. Three-dimensional reconstruction of the CT scan can add another layer of comprehension to the pathological anatomy5. After review of the imaging and with a carefully considered indication, these cases are best performed in a biplane cardiac catheterisation laboratory, with on-site access to thoracic and cardiac surgical expertise.
Although traditionally coils have been used to occlude PAVM, the size of the vessels involved is usually more effectively dealt with using vascular occlusion plugs such as St. Jude’s AMPLATZER™ vascular plug range (St. Jude Medical, St. Paul, MN, USA)6. These or a similar range of devices should be available in any catheterisation laboratory taking on these cases.
The usual practice is to perform selective angiography in the affected pulmonary artery branch. Identification of any proximal branches which supply normal lung is essential in order to avoid arterial occlusion of normal segments. Usually only the arterial supply to the fistula is occluded; however, some operators may consider occlusion of both the supplying arterial vessel and the pulmonary vein draining the lesion, particularly in complex lesions7. Occlusion devices are chosen using a sizing strategy that takes into account the local anatomy and also the characteristics of the device. This strategy will vary depending on anatomical characteristics and the characteristics of the chosen occlusion device. As the vessels involved are often very distensible, a device size of between 120% and 150% should be used8. When plugs are being implanted via long sheaths, the sheath size should accommodate the need for test angiograms during device deployment9,10 (Figure 2).
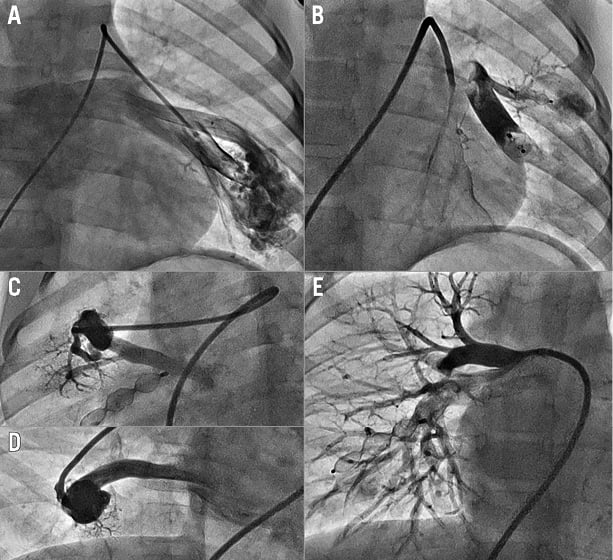
Figure 2. Occlusion of multiple large PAVM in a six-year-old child. A) Angiography before occlusion of a complex LLL PAVM with two major entry points, both of which are seen to be occluded on an angiogram in panel B. This patient had two further PAVM in his right lung (C&D). These were also occluded (E). He presented with severe desaturation and failure to thrive. Following interventional treatment his saturations increased from 76% to 96% and his finger clubbing had partially resolved within six months
Challenges in these cases may include avoidance of normal lung branches, dealing with multiple entry and exit points and damage to the fragile vessel wall of the malformation. As the majority of macrovascular PAVM lie at the periphery of the lungs (the most common site is in the left lower lobe [LLL]), damage to the sac of the PAVM by wire or sheath perforation may lead to a haemothorax (Figure 1).
Follow-up after the occlusion should include clinical monitoring and interval cross-sectional imaging, due to a tendency of even macrovascular PAVM to recur. Pleuritic chest pain is common in the two to three days after the procedure as a consequence of infarction of some normal and abnormal lung tissue. There may be an associated development of pleural effusions. This should be managed conservatively where possible2.
Conclusion
Diagnosis and management of PAVM is complex and often involves multiple teams due to the frequent association with other conditions, particularly HHT. Most symptomatic or prognostically important macrovascular lesions can be successfully managed using interventional techniques with good reported outcomes; however, large studies are lacking. The management of microvascular PAVM is mostly medical with the emphasis here on changing the constitution of the blood flowing to the pulmonary arteries by hepatic recovery or transplant, or by completion of the Fontan circulation.
Conflict of interest statement
The authors have no conflicts of interest to declare.

