A palliative Potts shunt is already used for the treatment of drug-refractory pulmonary arterial hypertension (PAH). We report on a transcatheter Potts shunt creation in a 19-year-old patient with PAH and Moyamoya syndrome. He was admitted with haemoptysis and end-stage right ventricular failure. The technique consisted of retrograde radiofrequency perforation of the descending aorta at the side of apposition to the left pulmonary artery for deployment of a 7 Fr, 22 mm long graft stent. Depicted are the final axial (Figure 1A) and coronal (Figure 1B) computed tomography (CT) images demonstrating the covered stent expanded to 7 mm, positioned between the left pulmonary artery (LPA) and descending aorta (DAO). The transcatheter approach was uneventful; the clinical symptoms improved based on an arterial right-to-left shunt with a significant difference of oxygen saturation measured between the upper and lower limb; the patient was discharged home. However, the implantation of the stent expanded to 7 mm did not avoid a second, eventually fatal haemoptysis. The detailed report is published in the Online Appendix. The step-by-step transcatheter technique of the Potts shunt created is illustrated in Online Figure 1, Online Figure 2, Online Appendix and Moving image 1-Moving image 8. The risk/benefit of creating a transcatheter Potts shunt needs to be confirmed in further studies.
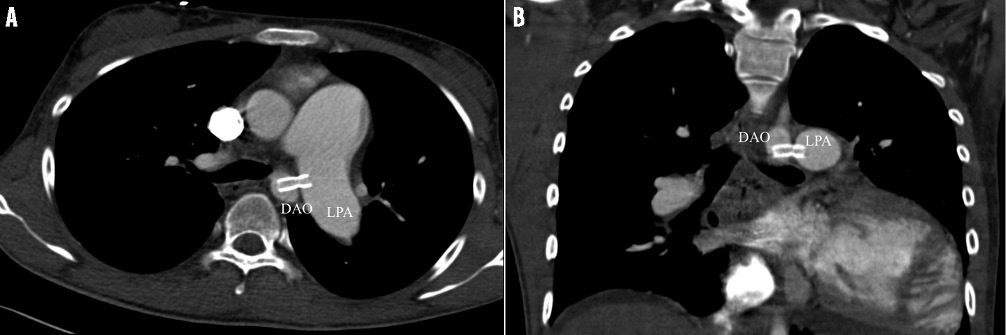
Figure 1. CT images of the Potts shunt. A) Axial CT image demonstrates the Potts shunt created by the expanded stent bridging the left pulmonary artery (LPA) and descending aorta (DAO). B) Stent position shown by CT image.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix. Transcatheter Potts shunt creation
Primary pulmonary hypertension (PPH) associated with Moyamoya (MM) syndrome was first described in 19901. Recently, the fatal outcomes of two paediatric patients with MM associated with pulmonary arterial hypertension (PAH) were described2. Pulmonary hypertension (PH) and MM associated with fibromuscular dysplasia of the pulmonary and systemic vascular systems was reported in two patients by Ou et al3. A common vascular denominator for both diseases is considerable although this hypothesis could not be proved. However, ubiquitous transforming growth factor beta superfamily signalling pathways could be playing such a role for both PAH4 as well as Moyamoya disease5,6. In MM disease, cerebrovascular accidents can appear as bleeding or cerebral stroke injury and take place spontaneously or following elective (even minor) surgical procedures7,8. On the other hand, progressive PAH causes overt right ventricular failure, recurrent syncope and even death9. Haemoptysis is also a worsening symptom and highly associated with sudden death10. Lung transplantation (LTx) is the final chance for selected patients with PAH, but to our knowledge LTx in patients with PAH in association with MM has not yet been reported.
PATIENT
A 19-year-old young man with longstanding PAH and familial MM syndrome was admitted with massive haemoptysis and severe RV dysfunction as a consequence of suprasystemic pulmonary hypertension. Ten years ago, when he was referred to our centre for the first time, MRI and cerebral angiography already showed bilateral narrowing of intracerebral vessels with massive MM typical collateral formation. Considering the rare association of MM with PPH1,2, he was chronically treated after several invasive, PAH-specific vasoreactive tests, which included the evaluation of the pulmonary flow reserve, as is standard in our centre11. Therefore, since the beginning of his symptomatic disease, he had been treated with amlodipine, sildenafil, acetylsalicylic acid and simvastatin; later on the therapy was expanded with spironolactone and bosentan, respectively. At the recent admission, his clinical condition had deteriorated to functional class IV with kidney injury and ascites; echocardiography showed severe tricuspid regurgitation, systolic bowing of the atrial and ventricular septum to the left heart side and pericardial effusion (Online Figure 1). An invasive diagnostic approach revealed a central venous pressure above 20 mmHg, pulmonary artery pressures of 145/79 mmHg accompanied by systemic arterial pressures of 120/73 mmHg; oxygen saturation of the superior caval vein was 38% despite continuous inodilator therapy with milrinone; aortopulmonary collateral vessels as one reason for haemoptysis were immediately looked for, but only two small collateral vessels were detected and closed by the coil technique. In addition, an atrioseptostomy was performed as a rescue therapy by the Brockenbrough technique followed by ballooning of the atrial septum. There was a slight clinical improvement at the expense of full body cyanosis with arterial oxygen saturations wavering between 75 and 85%. Therefore, transcatheter reverse Potts shunt creation together with re-closing of the atrial communication was considered and weighed against a surgical shunt approach in the context of the MM syndrome with a high risk for cerebral bleeding or stroke formation.
CREATION OF A REVERSE POTTS SHUNT
The technique to create a transcatheter reverse Potts shunt was based in part on the experiences published by Esch et al in patients with PAH12 and on an animal study by Sabi et al13. The step-by-step transcatheter approach is depicted in Online Figure 2A-Online Figure 2F, and additionally illustrated by Moving image 1-Moving image 8. After obtaining written consent from the patient and his parents, cardiac catheterisation was performed as a compassionate therapy guided by the ethical rules of the JLU-Giessen institution. The transcatheter approach was performed under conscious sedation, but with anaesthesiological and surgical stand-by. Following local anaesthesia, the left and right femoral veins and arteries were cannulated: 5 Fr sheaths were placed in left-sided and 9 Fr sheaths in right-sided vessels. Through the left-sided sheaths, 5 Fr pigtail catheters were advanced in the left pulmonary artery (LPA) and descending aortic arch (DAO) for diagnostic reasons. Guided by previously performed MRI pictures, in which DAO and LPA disposition was classified as Type I14, the relationship of both vessels was delineated by simultaneous angiographies of LPA and DAO (Online Figure 2A, Moving image 1); additionally, an Amplatz 25 mm snare (ev3/Covidien, Plymouth, MN, USA), was positioned in the LPA and through the short arterial 9 Fr sheath a 7 Fr long sheath (Cook, Copenhagen, Denmark) in the DAO. Thereafter, a 5 Fr Judkins catheter front-loaded with a Nykanen radiofrequency (RF) perforation wire (Baylis Medical [BMC], Montreal, QC, Canada) pre-mounted with a BMC coaxial injectable catheter was advanced through the 7 Fr long sheath and placed at the selected perforation site on the left lateral wall of the aorta in the immediate vicinity of the LPA (Online Figure 2B, Moving image 2). The visible tip of the Nykanen RF perforation wire could be easily advanced through the DAO-LPA vicinity using energy of 14 watts applied for 3 sec (Moving image 3). The HF wire was advanced together with the coaxial injectable catheter for stable placement in the distal LPA. The HF wire was exchanged for a Hi-Torque Extra S’port 0.014”, 300 cm coronary guidewire (Abbott Vascular, Santa Clara, CA, USA) with the coaxial catheter still in place. Then, the coaxial and Judkins catheters were replaced by a catheter with a hydrophilic coating on the shaft and a low-profile balloon (3×60 mm) enabling high crossability (Pacific Plus; Medtronic, Minneapolis, MN, USA). Pushing the long balloon and capturing the wire by the pre-positioned vascular snare, the balloon catheter was easily moved forward, even through the aortic-left pulmonary arterial walls, without any hindrance and verification of bleeding (Moving image 4). Thereafter, the 7 Fr long sheath was advanced through the vessel walls by inflating the low-profile balloon so that the taper of the balloon engaged the end of the long sheath (Online Figure 2C, Moving image 5). As already described by Esch et al11, “the balloon-sheath complex was then advanced into the PA by simultaneous pushing on the long sheath and pulling on the snared wire tip, with the balloon effectively functioning as a dilator for the sheath”. With the long sheath in the LPA, the balloon was deflated, removed, and replaced by a 7×22 mm covered stent (Atrium Medical, Hudson, NH, USA). The stent was positioned to bridge the LPA and DAO walls and the desired position confirmed by DAO and LPA angiographies (Online Figure 2D, Moving image 6). The part of the stent in the LPA was partially unsheathed and inflated (Online Figure 2E). Balloon inflation was continued by further retraction of the sheath (Moving image 7). When the stent was completely uncovered the stent was fully expanded by inflation pressure of 14 atm; the balloon was deflated and carefully removed, leaving the right-left shunting stent within the aortic and LPA walls (Online Figure 2F). Considering the 7 mm diameter of the covered stent in the aortic lumen of 15 mm, obstruction of the aortic lumen was excluded by angiography of the descending aorta (Moving image 8) and pressure measurement by retracting the pigtail catheter. After uneventful creation of the reverse Potts shunt, the total body oxygen saturation decreased to less than 70%. Therefore the atrial communication created one week earlier was closed by a 12 mm Amplatzer ASD occluder (St. Jude Medical, St. Paul, MN, USA). The arterial oxygen saturation increased to 87-93% at the upper limb and remained in the region of 70% at the lower limb. The fluoroscopy time of the Potts shunt creation and ASD closure together was 25.5 min in the anterior-posterior, and 5.3 min in the lateral projection. Two days later his clinical condition improved dramatically and the thoracic CT revealed a sufficient stent position (Figure 1A, Figure 1B); the patient was discharged home continuing with his PAH-specific drugs as at admission, expanded with digoxin and carvedilol to keep the heart rate less than 90/min for prolonging the left ventricular diastolic filling time. However, during the review process the patient again suffered a haemoptysis at home and died in a nearby intensive care unit.
Discussion
The creation of a Potts shunt has been reported to be a reasonable alternative treatment strategy instead of lung transplantation15,16. This procedure comprises the surgical or transcatheter construction of a connection between the left pulmonary artery and the descending aorta that allows right-to-left shunting such as in a patient with a patent ductus arteriosus-related Eisenmenger syndrome15-20. An update on the first 24 patients with a palliative Potts shunt for the treatment of drug-refractory PAH has been published by Baruteau et al16. Based on their experience with prolonged survival and dramatic, long-lasting improvement in functional capacities, the authors put forward the Potts shunt as a first surgical or interventional step in the management of children with severe, drug-refractory PAH, leaving the door open for further lung transplantation, if needed. However, in the absence of a patent arterial duct16,20, the implementation of such a shunt required an open heart surgery that carries a high periprocedural risk in these severely ill patients. A new transcatheter approach to solve this issue was recently published by Esch et al12. In three of four reported patients, the transcatheter approach utilising a transseptal needle was technically successful. One patient died of uncontrolled haemothorax during the procedure. However, the three successful procedures were demanding: one patient needed a second telescopic stent to prevent stent displacement, and two patients experienced balloon burst, one of them with detectable controlled bleeding. Considering the published experience of the Boston group12 and the transcatheter techniques described in animal models13, we favoured a retrograde RF perforation of the DAO at the side of apposition to the LPA for final placing of a covered stent as a shunt.
The hypotheses for our reported approach were as follows:
1.MRI imaged type I relationship of the DAO and LPA, with a distance of less than 4 mm in the landing zone between both vessels14, should be present to minimise a false direction of the energetic RF wire, and should allow the placement of an available premounted graft stent with a length of 22 mm.
2.Radiofrequency rather than mechanical transseptal Brockenbrough needle perforation of the neighbouring DAO-LPA walls should make the technique in total quite safe. Based on our institutional experience of perforating an atrial septum or Fontan conduits, we were convinced that the more traumatic needle technique is neither necessary for this indication nor less critical for the initial perforation of the native vessels. The bleeding risk by RF seemed lower because of a stable positioning of the RF wire and coaxial cable within the Judkins right and Cook long sheath ensemble at the limited area of the landing zone between both vessels. In addition, there should not be an “uncontrolled” need of mechanical force to perforate the vessels as in the RF technique, or the risk of pushing away the LPA wall before perforation as might be assumed using the needle technique. Further, the RF potential to coagulate the tissue at the minimal perforation sites should prevent extravasation of blood.
3.Considering the reported animal data utilising the RF technique for creating an aortopulmonary shunt by Sabi et al13, we were convinced that the risk of bleeding should be minimised by utilising the described balloon-sheath ensemble procedure instead of a regular long sheath with the conical end to enter the lumen of the LPA. Again, based on our experience with transcatheter creation of a fenestration in a Fontan circulation with an extracardiac conduit where we have a great deal of experience with such a balloon-sheath technique, here in particular we used a very long, low-profile balloon catheter. This balloon catheter is the one with the best crossability potential to allow advancement of the balloon through the vessel walls followed by the long 7 Fr sheath over the balloon already positioned between the vessels and inflated. For safety reasons and to simplify crossing the long sheath through the vessel walls, we did not choose a short 20 mm but rather a 60 mm long balloon with a width of 3 mm, slightly more than the diameter of the 7 Fr long sheath.
The principal goal of the approach is to create a right-left shunting Potts shunt, changing the cardiac pathophysiology into that of duct-related Eisenmenger’s physiology. Ideally, the pulmonary circulation should be unloaded consecutively, a failing RV decompressed, and the systemic circulation simultaneously supported but not at the expense of an induced cyanosis of the coronary and cerebral perfusion due to an insufficient left ventricular preload. Therefore, the Potts shunt width remains a compromise between an unrestrictive arterial communication and a significant, but still acceptable difference in the oxygen saturation between the upper and lower limbs. In addition, the immediate and long-term adaptation to the newly created parallel circulation has to be considered. Therefore, there is room for innovation from a flow-reducing to a re-dilatable graft stent, to a valved conduit16. Here, we used a 7 mm graft stent taking into consideration the data from Esch et al12, and also based on our experience in an adult patient, in whom a 13 mm conduit needed to be reduced to almost 7 mm by transcatheter flow restriction18, and later, after further improvement, with the creation of a valved conduit by percutaneous implantation of a Melody® valve (Medtronic, Minneapolis, MN, USA) within the shunt (not yet published).
Despite the fact that we could not avoid the sudden death of our young patient by the uneventfully performed Potts shunt, we are convinced that the creation of a Potts shunt expands the option for drug-resistant pulmonary hypertension perhaps if it is performed earlier. However, the risk/benefit of the transcatheter Potts shunt creation needs to be confirmed in further studies.
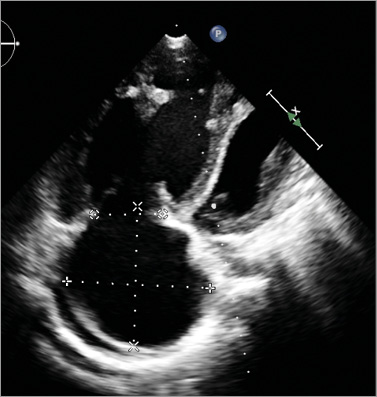
Online Figure 1. Echocardiography in four-chamber view shows the enormously dilated right atrium and ventricle and its influence on the left cardiac side; pericardial effusion is seen behind the right atrium.
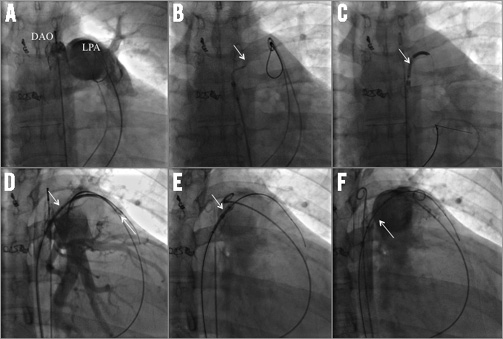
Online Figure 2. Simultaneous angiography of the descending aorta (DAO) and left pulmonary artery (LPA). A) Type I relationship of both vessels (Moving image 1); in addition to the pigtail catheters placed in the DAO and pulmonary artery, a snare positioned in the LPA and coils in two small aortopulmonary collateral vessels are depicted. B) The arrow marks the tip of the high-frequency catheter, which is positioned through a 5 Fr Judkins right catheter and 7 Fr long sheath (Moving image 2). Moving image 3 shows the smooth perforation of the DAO and LPA wall by the radiofrequency wire, its advancing and floating in the LPA. C) The arrow marks the inflated 60×3 mm balloon catheter, which allows advancing the 7 Fr long sheath from the DAO to the lumen of the LPA. In addition, Moving image 4 shows the DAO angiography without any contrast leakage delineating the low-profile 6 cm balloon catheter advanced through a 7 Fr long sheath and already crossed to the LPA. Moving image 5 demonstrates the exact vessel wall connection by the waist during balloon inflation; the snared pulmonary wire end was pulled down in the right ventricle. D) The crossed long sheath is depicted, which is already positioned within the lumen of the LPA; one arrow marks the tip of the sheath, the other the graft stent within the long sheath. Moving image 6 shows angiographies of LPA and DAO demonstrating the stent position provided and the closed neighbourhood of both vessels; additionally, any detectable bleeding by leaving contrast medium could be excluded. E) The arrow demonstrates the partially uncovered and inflated graft stent during retraction of the long sheath from the LPA to DAO (Moving image 7). F) Pulmonary angiography with simultaneous descending aortogram after covered stent placement demonstrates the newly created Potts shunt without any aortic obstruction and leakage of contrast medium (Moving image 8).
Online data supplement
Moving image 1. Simultaneous LPA-DAO.
Moving image 2. DAO-LPA vicinity.
Moving image 3. RF perforation.
Moving image 4. Balloon crossing.
Moving image 5. Balloon inflation.
Moving image 6. 7 Fr sheath positioning.
Moving image 7. Stent expansion.
Moving image 8. LPA-DAO cine after Potts shunt.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Simultaneous LPA-DAO.
Moving image 2. DAO-LPA vicinity.
Moving image 3. RF perforation.
Moving image 4. Balloon crossing.
Moving image 5. Balloon inflation.
Moving image 6. 7 Fr sheath positioning.
Moving image 7. Stent expansion.
Moving image 8. LPA-DAO cine after Potts shunt.

