Abstract
Pulmonary vein stenosis (PVS) is an uncommon but devastating complication of atrial fibrillation (AF) ablation. Patients are often misdiagnosed due to non-specific symptoms and the challenges of visualising the pulmonary veins on standard chest imaging. Delays in treatment result in worsening symptoms and pulmonary venous occlusion. The optimal method of intervening for PVS has not been well established. Restenosis after successful intervention is common, warrants active surveillance, and is the focus of research into prevention and management strategies. In this article we review the existing literature on PVS, and discuss our own experience in managing patients with severe PVS.
Introduction
The incidence of pulmonary vein stenosis (PVS) has decreased substantially with modification of atrial fibrillation (AF) ablation techniques. As a result, many providers now forego post-ablation surveillance CT imaging, which was previously the standard of care1. Unfortunately, decreased screening delays diagnosis and can result in more advanced disease with increasingly severe pulmonary vein (PV) fibrosis and intraparenchymal lung damage. Early and accurate identification of PVS, followed by appropriate invasive management, is critical to improving patient outcomes. This article will discuss the causes, presentation, and management of PVS by reviewing the existing literature. We will also discuss our own experiences in managing these patients.
Ablation factors
The triggers which initiate AF typically originate from muscle sleeves within the PV ostium. Consequently, the first ablation techniques involved radiofrequency energy applied directly within the venous os. This resulted in an unacceptable rate of PVS, up to 46% in early series, and drove adoption of newer techniques, including antral ablation and wide area circumferential ablation1,2. The integration of intracardiac echocardiography has further reduced the incidence of post-ablation PVS by aiding in identifying the true PV ostium during ablation1. However, PVS continues to complicate 1.3-3.4% of AF ablations, and methods to prevent PVS remain an active area of research1,3.
Symptoms
Symptoms vary depending on the chronicity, number of affected veins, severity of stenosis, and physical activity level of the patient. When routine screening is employed, asymptomatic patients make up between 4-8% of severe PVS cases1,4,5. At our centre, asymptomatic patients with significant stenosis undergo invasive testing. If angiography confirms severe stenosis, intervention is performed to prevent progression to PV occlusion. At centres without routine screening, patients come to attention after they become symptomatic1. Subtle limitations can begin within days of the ablation, and fulminate symptoms may manifest anywhere from one to 12 months later2,6. The most common complaints are non-specific and include exertional dyspnoea, chest pain, pleuritic pain, fatigue, cough, flu-like symptoms, and decreased exercise tolerance. Haemoptysis is perhaps the most specific manifestation, but occurs only in a minority of patients2,5.
Diagnosis
Cardiac computed tomography (CT), and to a lesser extent cardiac magnetic resonance imaging (MRI), provide the best imaging of the PVs1,4. Cardiac MRI avoids radiation; however, CT offers superior spatial resolution with the ability to perform 3D reconstruction with generation of en face views for accurate determination of the luminal diameter (Figure 1A)4. The CT must be performed with a specific protocol timed for enhancement of the PVs. We have treated several patients in whom the diagnosis of PVS was delayed after an improperly timed CT failed to visualise the PVs. Additionally, chest imaging frequently demonstrates lung parenchymal congestion or subpleural infarction, which can masquerade as a pneumonia or malignancy (Figure 1B).
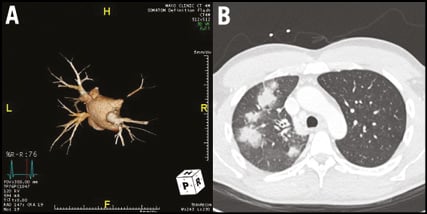
Figure 1. Imaging findings. A) & B) Three-dimensional reconstruction of a pulmonary vein protocolled CT scan demonstrates near total occlusion of the right superior pulmonary vein and moderate stenosis of the left inferior pulmonary vein. Chest imaging from the same patient reveals patchy right upper lobe consolidation due to venous congestion.
A significant number of patients experience delays in care due to decreased screening, non-specific symptoms, the need for dedicated PV imaging, and misdiagnosis. Many patients are treated for pneumonia or bronchitis, or undergo invasive assessment with bronchoscopy or biopsy for presumed lung malignancy2. At our centre, a third of patients were initially given a diagnosis other than PVS, resulting in delayed care6. These delays result in progressive symptoms, increasingly severe stenosis or occlusion, dense fibrosis, and lung parenchymal damage.
Treatment
Briefly, femoral access is established with 8 Fr and 10 Fr sheaths to facilitate passage of an intracardiac echocardiogram (ICE) catheter and transseptal sheath. A 6 Fr multipurpose catheter is advanced through the transseptal sheath and advanced to the affected PV. Therapeutic heparinisation is maintained to an activated clotting time (ACT) of 250-350 seconds. Contrast injections are used to confirm severe stenosis (Moving image 1). A guidewire is passed into the vein and the multipurpose catheter advanced. A balloon is positioned within the area of stenosis and multiple inflations are carried out. In cases with persistent stenosis, a stent may be deployed (Moving image 2). Stents are sized to the largest achievable diameter4.
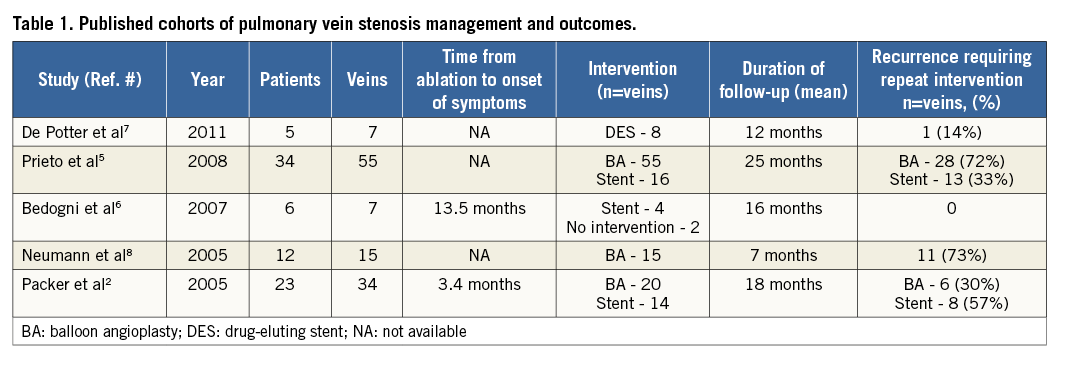
Published outcomes data on the efficacy of PV interventions are limited to small case series with mixed results (Table 1). Restenosis after a successful intervention has been reported in between 30 to 73% of veins treated with balloon angioplasty (BA), and 0 to 57% of veins treated with stenting2,5,7,8. The largest case series included 34 patients with 55 stenotic veins, and reported a 72% risk of restenosis after BA, and 33% risk of restenosis after stenting (HR 2.6, p=0.003)5. Larger series examining outcomes after intervention are ongoing and will add additional data on the optimal initial management strategy.
The choice of index BA versus stenting varies by institution. When stents are deployed, the largest acceptable stent size, usually 10 mm, should be selected as there are data to suggest long-term patency is superior with larger stents8. In tightly stenotic veins, the stent diameter may be limited by fibrosis. In these cases, or in lesions involving bifurcations, drug eluting stents (DES) may be employed. There are only anecdotal data on the use of DES in treating PVS7. Our own experience with coronary DES has been disappointing, with the majority of veins experiencing restenosis. This is probably due to the persistently small venous diameter and pre-existing advanced fibrosis.
After hospital discharge we follow patients with repeat imaging at three-month intervals for the first year. Tight surveillance is crucial, as PV restenosis can occur abruptly and progress rapidly. All patients must be instructed to seek care immediately if they experience recurrent symptoms, as urgent intervention may be warranted.
Long-term medical therapy
There are no controlled data on optimal adjunctive therapy, and use of antithrombotic therapy varies widely by institution2,4,6,8. We load all patients with 300-600 mg clopidogrel prior to intervention and continue 75 mg daily indefinitely. We advise life-long therapeutic anticoagulation with coumadin, regardless of whether the patient had a successful ablation. The goal is to minimise stent thrombosis, particularly as the PVs are a low-pressure system, which may result in venous occlusion, systemic embolisation, or stroke.
Discussion
The diagnosis of PVS is difficult due to non-specific symptoms and the need for dedicated chest imaging. Patients with severe stenosis warrant invasive management to prevent PV occlusion and lung parenchymal damage. Published data are heterogeneous but suggest less restenosis when large stents are used for the index procedure. Restenosis is common regardless of the initial management approach and warrants active surveillance.
Translational data have demonstrated neointimal hyperplasia as one of several mechanisms underlying the development of PVS3,7. The role of antiproliferative agents such as drug-eluting stents in treating PVS remains poorly defined. Large-diameter drug-coated peripheral stents are not yet widely available, but may provide superior long-term patency when compared to coronary DES or larger peripheral BMS. Additional studies into the underlying mechanism of PVS, as well as possible therapies both to prevent and to treat PVS are ongoing3.
Conclusion
Despite advances in ablation techniques, PVS remains a highly morbid complication of AF ablation. Lack of widespread post-ablation screening coupled with non-specific symptoms often leads to misdiagnosis and late presentation. Delayed intervention risks increasing fibrosis, venous occlusion, and venous congestions with lung infarction; therefore, all patients with severe PVS should undergo early invasive management regardless of the presence of symptoms.
Data from small cohorts on the efficacy of BA and stenting are heterogeneous but suggest stenting with large-diameter stents may be superior. A large cohort study of PVS interventions is ongoing and will add significantly to our understanding of long-term outcomes.
Conflict of interest statement
D. L. Packer provides consulting for Abiomed, Biosense Webster, Inc., Boston Scientific, CardioDX, CardioFocus, CardioInsight Technologies, InfoBionic, Inc., Johnson & Johnson Healthcare Systems, Johnson & Johnson, MediaSphere Medical, LLC, Medtronic CryoCath, Sanofi-Aventis, Siemens, St. Jude Medical, and Topera Medical. D.L. Packer received no personal compensation for these consulting activities. He receives research funding from the AHA Foundation Award, Biosense Webster, Boston Scientific/EPT, CardioInsight, CardioFocus, Endosense, EpiEP, EP Rewards, Hansen Medical, Medtronic CryoCath LP, NIH, St. Jude Medical, and Siemens. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Contrast angiography demonstrates 99% stenosis of a left superior pulmonary vein.
Moving image 2. Following successful deployment of a 4×23 mm drug-eluting stent, the stenosis has resolved.
Supplementary data
To read the full content of this article, please download the PDF.
Contrast angiography demonstrates 99% stenosis of a left superior pulmonary vein.
Following successful deployment of a 4×23 mm drug-eluting stent, the stenosis has resolved.

