CASE SUMMARY
BACKGROUND: A 33-year-old adult with complex congenital heart disease and severe cyanosis presented to us with increasing symptoms of hyperviscosity, decreasing exercise tolerance and a thromboembolic complication.
INVESTIGATION: Physical examination, laboratory investigations, ECG, transthoracic echocardiogram, cardiopulmonary exercise testing, angiography.
DIAGNOSIS: Subvalvular and valvular pulmonary stenosis due to a calcified pulmonary valve.
TREATMENT: Amiodarone, angiotensin-converting enzyme inhibitor, beta-blockers, percutaneous pulmonary balloon valvuloplasty, warfarin.
KEYWORDS: congenital heart disease, cyanosis, palliation, pulmonary valvuloplasty
PRESENTATION OF THE CASE
A 33-year-old man with double-inlet left ventricle, ventricular septal defect, rudimentary subaortic right ventricular outlet chamber, L-transposition of the great arteries, subvalvular and valvular pulmonary stenosis introduced himself to our adult congenital heart disease clinic in 2008. He worked as an electrical engineer and had recently obtained his PhD. He reported a decreased exercise capacity with dyspnoea on exertion. On examination he showed signs of severe cyanotic heart disease with an oxygen saturation of 75% at rest and clubbing of the fingers and toes. On auscultation, a 4/6 systolic murmur over the left sternal border radiating throughout the chest was audible. Laboratory findings showed secondary erythrocytosis with a haematocrit of 61%. Because of the stable clinical condition and the balanced haemodynamic situation we decided to pursue a conservative follow-up.
In 2010 the patient presented with an acute scotoma on his right eye. Magnetic resonance imaging showed an ischaemic lesion in his right central retinal artery. The scotoma was interpreted as a thromboembolic complication triggered by dehydration during a common cold and iron deficiency (ferritin at this point of time was decreased to 20 ng/ml and haematocrit had increased to 71.3%). Following adequate rehydration, a phlebotomy was performed and iron supplementation as well as an aspirin therapy with 100 mg/day was started. A cardiopulmonary exercise test revealed a reduced exercise capacity with a low peak oxygen consumption (14 ml/min/kg) and significant desaturation with exercise (44%). A transthoracic echocardiogram showed a slightly impaired ventricular function, a mild fibrous subpulmonary obstruction and severe pulmonary stenosis with a calcified pulmonary valve (diameter of 25 mm) with little antegrade flow across on colour flow Doppler (Figure 1). Angiography also showed restricted opening and doming of the calcified pulmonary valve (Figure 2).
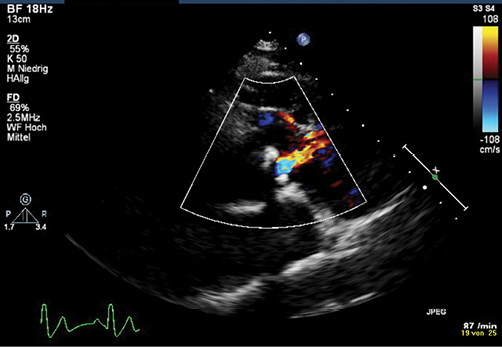
Figure 1. Transthoracic echocardiography showing little flow across the doming and calcified pulmonary valve on colour flow Doppler1. (With kind permission of Springer Science+Business Media).
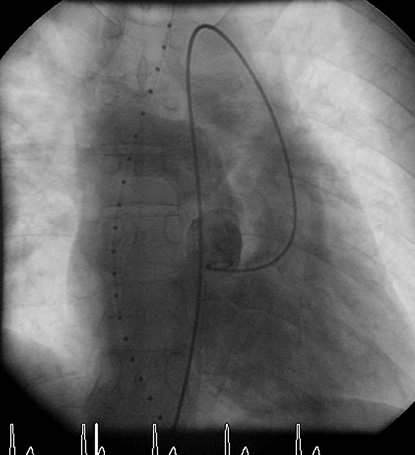
Figure 2. Angiography demonstrates the restricted opening with doming of the calcified pulmonary valve.
How would I treat?
THE INVITED EXPERTS’ OPINION
First of all, complex adult congenital heart disease does not fit into a quiz format, because decisions are made step by step within the congenital Heart Team and are based on clinical and diagnostic findings on the one hand and on the experience of the team on the other. In this area of adult congenital heart disease, treatment strategies are often an extrapolation of paediatric protocols, while actual experience is limited and evidence lacking.
The information on cardiac function in this particular case seems to be adequate for decision making, but information on pulmonary vascular anatomy and physiology is lacking. A perfusion scan and measurement of pulmonary vascular resistance would be informative.
Treatment would consist in any case of optimal conservative measures including, e.g., haematocrit management, anti-aggregation, anticoagulation and antibiotic prophylaxis.
Considering further treatment, shared decision making with the patient would be in order, as he seems capable of understanding the complex decision making associated with his particular situation.
A limited increase in the pulmonary blood flow should result in an improved oxygenation. This can be accomplished by creating an aortopulmonary shunt, e.g., a modified Blalock-Taussig shunt through a lateral thoracotomy. Alternatively, limited dilatation or stenting of the pulmonary valve (restoring the natural equilibrium with some pulmonary stenosis) could be considered, but the calcification would form a risk of uncontrollable dilation or of unreliable fixation of a stent or stented valve. Too much stenosis relief might augment pulmonary flow to such an extent that cardiac failure is likely to occur. Percutaneous intervention contains a risk of failing palliation, and an alternative approach should be considered in case of an unacceptable result.
If the pulmonary vascular condition would permit, a bidirectional cavopulmonary shunt can be considered with the option of permanent palliation, secondary total cavopulmonary connection or, if all of these fail, cardiac transplantation2,3.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
Neonates with functional single ventricles have a very high morbidity and mortality. Survival is only 30% for the first year of life and only about 30% of patients with a univentricular heart reach the age of 16 years4,5.
Most patients with a univentricular heart suffer from dyspnoea, cyanosis, and reduced exercise tolerance. Survival into late adulthood, without surgical repair, is rarely reported6,7.
The prognosis for patients with either one or two ventricles managed with a single ventricle approach has improved considerably over the past two decades. At this stage, the treatment is focused on the optimising of the anatomy and physiology for a subsequent Fontan procedure. However, attention should focus on the ratio of the pulmonary and systemic perfusion (Qp/Qs). Besides this, the prognosis depends on the pulmonary vascular resistance, the function of the ventricle and of the atrioventricular valves8.
A balanced haemodynamic condition, good ventricular function and adequately functioning atrioventricular valves can allow good quality of life and a good functional capacity, even in an unoperated patient8,9.
In the case presented, long-term survival of this patient was probably achieved because of a left-type ventricle with a balanced haemodynamic condition. The presence of competent atrioventricular valves and only slightly impaired valvular function was associated with an almost uncomplicated growing-up of this patient. If severe pulmonary stenosis or subpulmonary stenosis is present, it will lead to severe oxygen desaturation. However, a deterioration of this condition represents a dilemma. The indication for a Fontan procedure may be highly questionable.
From our point of view, the purpose of a treatment is to restore a balanced haemodynamic. Surgical repair is controversial because of an expected significant operative risk in repeated interventions.
A transcatheter pulmonary valve replacement has proved to achieve satisfying short- and medium-term results and is described as a safe and efficient procedure. Several authors have detailed low numbers of periprocedural complications10,11. Although well accepted, it is limited to the management of right ventricular outflow tract (RVOT) <22 mm in diameter10,12.
For patients with larger RVOT diameters, Boudjemline and colleagues recently reported their convincing results of valve replacement. Using the Russian dolls technique and/or the PA jailing technique they prepare the RVOT for transcatheter valve implantation13.
The Russian dolls technique is appropriate for patients where the RVOT and the pulmonary artery are large. The RVOT region is minimised by deploying multiple stents with decremental diameters. Finally, the right Melody® heart valve (Medtronic, Minneapolis, MN, USA) can be implanted.
When the diameter of a pulmonary artery is <22 mm and the RVOT is >22 mm, stenting is started from that pulmonary artery to the RVOT with overlapping uncovered stents13.
It has to be mentioned that a certain degree of pulmonary stenosis is physiologically desirable to prevent pulmonary overcirculation.
Nevertheless, an appropriate medical treatment should be carried out regardless of the valve replacement, including standard heart failure treatment and anticoagulants to prevent thromboembolism.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The options of increasing the pulmonary blood flow and hence improving the functional status of our patient were extensively discussed. Surgical procedures like placement of an aortopulmonary shunt or the Fontan operation with an extra-cardiac conduit were judged to be high risk due to the severe chronic cyanosis. A percutaneous pulmonary valvuloplasty was felt to be of acceptable risk bearing in mind that a small balloon to pulmonary valve ratio may not improve pulmonary blood flow and a high ratio may result either in significant pulmonary regurgitation or massive flow resulting in pulmonary hypertension in due course. Pulmonary valvuloplasty was performed with two inflations using a VACS III 20 mm balloon (Osypka AG, Rheinfelden, Germany). Following the intervention the valve showed a better opening (Figure 3A, Figure 3B). The systolic pulmonary artery pressure increased from 12 to 35 mmHg, and the peak-to-peak gradient across the pulmonary valve decreased from 90 to 65 mmHg. Oxygen saturation improved from 72% to 92% in room air. Because of slight dyspnoea and rales on pulmonary auscultation, the patient received diuretics for the subsequent six weeks.
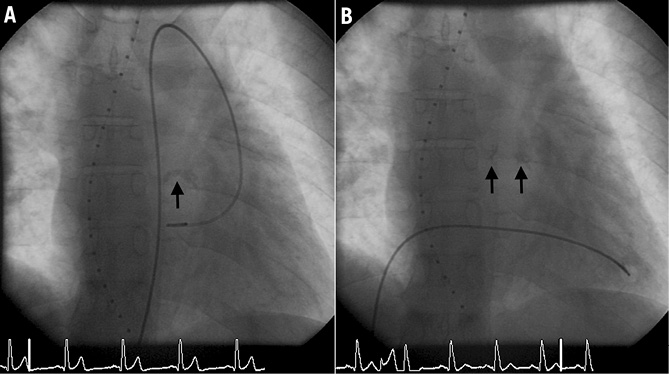
Figure 3. Fluoroscopy at end systole demonstrating the narrow, calcified pulmonary valve (A), which opens better after intervention (B).
Six months following the intervention the patient was pink with an oxygen saturation of 93%. Erythrocytosis and hyperuricaemia disappeared within three months. The electrocardiogram (ECG) showed a marked increase of QRS duration to 180 ms compared to 120 ms before intervention. Six-minute walking test as well as cardiopulmonary exercise testing demonstrated an improvement of exercise capacity with only mild desaturation on exercise (Table 1). Colour Doppler echocardiography showed a broad systolic jet across the pulmonary valve with minimal regurgitation (Figure 4).
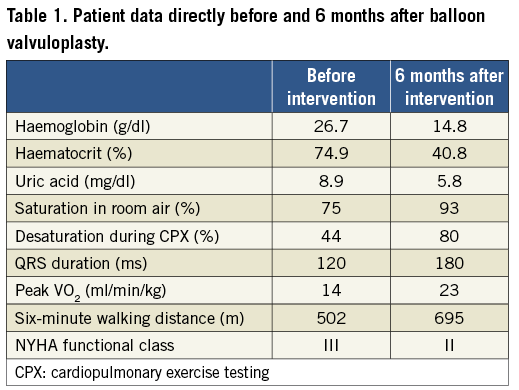
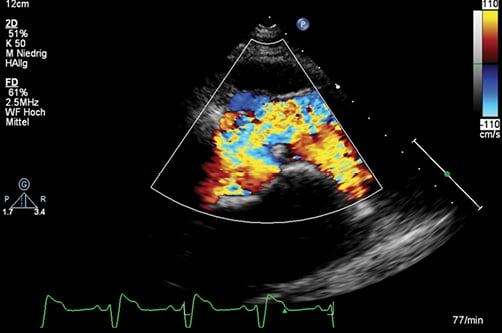
Figure 4. A broad antegrade flow jet following balloon dilatation is seen on transthoracic echocardiography1. (With kind permission of Springer Science+Business Media).
At the end of 2011 –almost a year after balloon valvuloplasty– the patient suffered from syncope while walking to work. Basic life support was provided by laymen, and the emergency physician detected atrial fibrillation with a ventricular rate of 180 bpm, which was successfully cardioverted into sinus rhythm. The patient recovered completely without neurological sequelae. Oral anticoagulation with warfarin and an anti-arrhythmic treatment with beta-blocker and amiodarone were initiated. Over the following 12 months the patient did well without cardiac symptoms. A current repeat cardiac catheter showed a Qp:Qs of 2.1:1 with normal pulmonary artery pressures and resistance.
Case conclusion
This case highlights the therapeutic dilemma of patients with the multisystem disorder of cyanotic congenital heart disease due to restricted pulmonary blood flow. Cardiac surgical procedures are of high risk in the adult, and experiences with interventional procedures to enhance pulmonary blood flow are limited. The short- and mid-term result of balloon valvuloplasty of a calcified stenotic pulmonary valve was satisfactory in our patient. Clinical symptoms of central cyanosis with marked erythrocytosis disappeared and exercise tolerance improved. However, life-threatening atrial fibrillation with high ventricular rates a year following the intervention might have been a consequence of increased pulmonary blood flow with volume loading of the ventricle.
Discussion
PULMONARY VALVULOPLASTY IN PATIENTS WITH COMPLEX CYANOTIC HEART DISEASE WITH SEVERE PULMONARY STENOSIS
Complex congenital cyanotic heart disease (CCHD) due to restricted pulmonary blood flow often requires surgical intervention in early childhood. This will either be the creation of a systemic-to-pulmonary artery shunt followed by complete repair or a definite palliation. Few patients survive into adulthood without operation. With increasing age it is likely that these patients suffer from symptoms related to increasing cyanosis. Pulmonary balloon valvuloplasty to palliate symptoms and alleviate hypoxaemia has been described only in very few adults with unrepaired CCHD14,15. However, detailed clinical follow-up is lacking. Balloon dilatation of calcified stenotic pulmonary valves in elderly adults with a structurally normal heart may be successful16. However, there are no such reports in adults with CCHD.
Clinical data on adults with complex congenital cyanotic heart disease following percutaneous pulmonary balloon valvuloplasty are scarce. In a case series of percutaneous interventions in adults with cyanotic heart disease, Book et al reported on a 33-year-old female patient with double-inlet left ventricle and subpulmonary and pulmonary stenosis14. After pulmonary valvuloplasty her exercise tolerance improved markedly. Two years later, however, she presented with NYHA III symptoms. A repeat cardiac catheter showed elevated ventricular end-diastolic pressures, which were interpreted as a result of an increased left-to-right shunt. Stümper et al described a 29.5-year-old patient with severe cyanosis due to transposition of the great arteries, ventricular septal defect and pulmonary stenosis15. A balloon to pulmonary annulus diameter ratio of 1:1 was chosen which improved saturations from 73% to 83% at follow-up. Balloon dilatation of calcified stenotic pulmonary valves in elderly adults with a structurally normal heart using balloon diameters according to the size of the pulmonary annulus can be successful16,17.
There are no data on long-term outcome after balloon valvuloplasty in this particular patient group. Despite the abolition of central cyanosis in our patient, the risk of life-threatening arrhythmias such as atrial tachycardia or atrial fibrillation based on a chronically hypoxaemic heart persists. It cannot be excluded that the volume load following the intervention was a trigger for these arrhythmias. ICD implantation was considered but not deemed mandatory, as no ventricular arrhythmia had been detected in our patient.
Conclusion
A calcified pulmonary valve in a patient with complex congenital cyanotic heart disease can be successfully dilated resulting in a significant clinical improvement. A smaller balloon diameter than used could have resulted in a more advantageous residual cyanosis with less volume load of the systemic ventricle and possibly less risk of life-threatening arrhythmias.
Conflict of interest statement
The authors have no conflicts of interest to declare.

