CASE SUMMARY
Background: A 45-year-old hypertensive male had presented with recent onset New York Heart Association (NYHA) class II angina and dyspnoea on exertion of six-weeks duration. The exercise treadmill test was strongly positive with significant ischaemic changes occurring in stage 1 of Bruce protocol. A percutaneous coronary intervention (PCI) to occluded obtuse marginal (OM) branch was performed. Later he developed hypotension with pericardial effusion.
Investigation: Physical examination, electrocardiography, 2D echocardiography, coronary angiogram.
Diagnosis: Distal coronary perforation.
Treatment: Percutaneous transcoronary glue injection.
Keywords: coronary perforation, cyanoacrylate glue, recurrent pericarditis.
PRESENTATION OF THE CASE
A 45-year-old hypertensive male had presented with recent onset NYHA class II angina and dyspnoea on exertion of six-weeks duration. The exercise treadmill test was strongly positive with significant ischaemic changes occurring in stage 1 of Bruce protocol. Angiogram showed a total occlusion of left circumflex artery and obtuse marginal branch (OMB) (Figure 1). The patient underwent PCI after an informed consent. The patient was given 600 mg clopidogrel one day prior and 5,000 U intravenous heparin was given at the start of the procedure. A Judkins left (JL) 3.5 guiding catheter (Cordis Corp., Johnson & Johnson, Miami Lakes, FL, USA) was used to hook the left main artery. As a soft coronary wire could not be negotiated through the lesion, a hydrophilic Crosswire® NT (Terumo Medical Corporation, Somerset, NJ, USA) was used to cross the lesion. The distal end of the guidewire was parked in a moderate sized OM branch. The lesion was stented with Bx Sonic™ (Cordis Corporation, Johnson & Johnson, Miami Lakes, FL, USA) 2.75×28 following predilatation. The stent was postdilated with a Powerline™ 3×10 balloon (Biosensors Interventional Technologies Pte Ltd, Singapore). The end result appeared satisfactory (Figure 2). Intravenous tirofiban (0.4 µg/kg/min over 30 min followed by 0.1 µg/kg/min continuous infusion) was given.
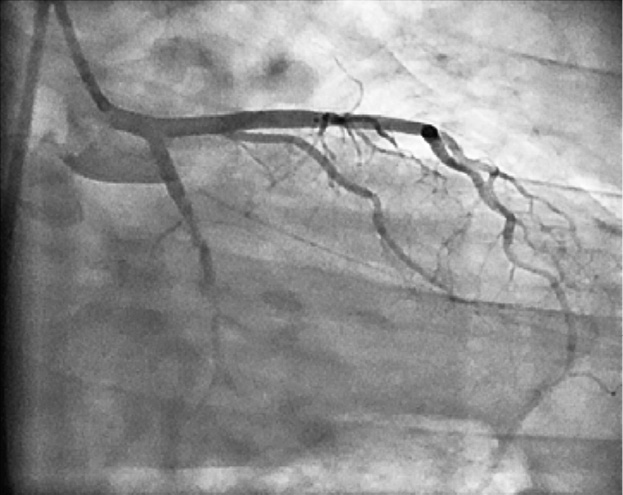
Figure 1. Left coronary artery (LCA) angiogram in right anterior oblique view with caudal angulation showing total occlusion of the left circumflex artery with retrograde filling of a good-sized obtuse marginal artery .
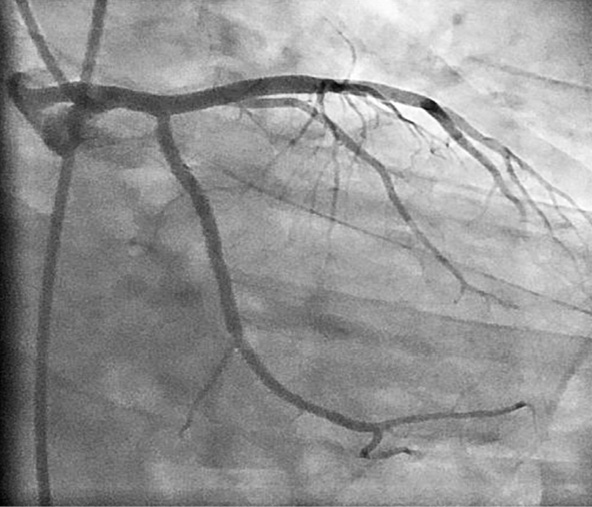
Figure 2. Coronary angiogram showing successful deployment of the stent with TIMI 3 flow attained at the end of the procedure.
The patient had hypotension two hours later, which was not associated with chest pain or any electrocardiogram (ECG) change. Echocardiography showed significant pericardial effusion with evidence of tamponade. Repeated pericardiocentesis with autotransfusion was done. Tirofiban infusion was stopped and 10 mg of protamine was used to reverse the action of the heparin. The pericardial effusion re-accumulated rapidly. Hence, the patient was taken to the catheterisation laboratory after two hours of failed conservative management. A coronary angiogram showed a patent stent and a type III perforation of the distal OM branch (Figure 3).
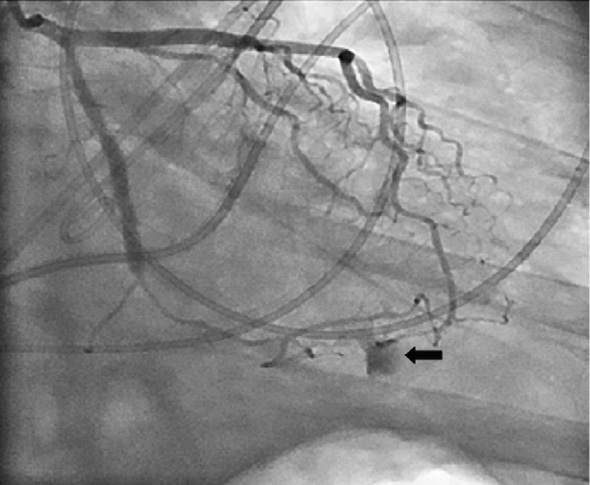
Figure 3. Coronary check angiogram showing the extravasation of contrast from the terminal branch of the obtuse marginal artery and a well deployed stent across the lesion.
How would I treat?
THE INVITED EXPERT’S OPINION
Coronary perforations (CP) are rare but feared complications of coronary interventions1-4. Treatment of guidewire-induced CP (GW-CP) is challenging because they may be associated with catastrophic clinical consequences if unsuspected or poorly managed. GW-CP are frequently a result of aggressive/prolonged procedures, stiff (chronic total occlusions) or hydrophilic (complex lesions) guidewires, and adjunctive treatment with glycoprotein IIb/IIIa platelet inhibitors (GPI1-4). Although GW-CP may initially have a benign angiographic appearance they frequently evolve as Ellis class III CP (extravasation jet through frank [>1 mm] perforation or cavity spilling) entailing adverse prognosis1.
If this complication is identified during the procedure, different “conservative” strategies may be implemented while the patient is haemodynamically stable. Classically, these include reversing the anticoagulation with protamine and prolonged balloon inflations1,3. Interestingly, these manoeuvres have been reported to be successful in >50% of patients3. However, if leakage persists, the only reliable therapeutic approach remains occlusion of the distal vessel. Currently available microcatheters are able to negotiate very distal and tortuous small vessels and allow selective injection of several substances (thrombin, collagen, polyvinyl-alcohol, Gelfoam, or even subcutaneous fat) directly at the target site3,5. In addition, different commercially available microcoils can be readily deployed to seal the leakage6,7. The price to pay is the occlusion of small distal vessels with relatively small enzymatic rises that favourably compares with the potential risk associated with cardiac tamponade. Of note, nearly fifty per cent of patients with type III CP eventually develop a myocardial infarction3.
However, faint signs of extravasation at distal vessels are easily missed during the procedure. Therefore, quite often GW-CP are only identified “a posteriori” as the result of haemodynamic derangement from cardiac tamponade. This occurred in the case presented by Senguttuvan et al in this issue of EuroIntervention. A time delay of two hours indicates a procedural-related complication but we have seen many cases where this complication was only clinically apparent >24 hours after the intervention, making clinical diagnosis much more challenging. In any case, this situation requires a high degree of clinical suspicion (echocardiography is required to confirm pericardial effusion) and urgent management. In the patient described by Senguttuvan et al, re-accumulation of pericardial fluid despite repeated pericardiocentesis constitutes a clear indication for definitive therapeutic measures once the conservative approach proved to be ineffective. Platelet transfusion remains yet another possibility due to prior GPI treatment but the severe haemodynamic derangement requires expedited treatment with a reliable intervention1-3.
At this stage, two interesting and striking angiographic findings are apparent from the still images provided by the authors. First, the coronary segment immediately distal to the stent shows a diffuse and severe reduction in calibre as compared with the post-intervention image. We have seen similar images in this setting secondary to associated coronary spasm or vasoconstriction due to haemodynamic instability. External lumen compression by an associated intramural coronary haematoma or by a “localised” pericardial effusion (the use of protamine and the requirement of multiple pericardiocentesis should be kept in mind) might be considered but appears highly unlikely. Second, and much more importantly, the angiographic image also suggests that a vessel, originating from the left anterior descending coronary artery, fills the distal marginal branch close to the site of GW-CP. Considering that collateral supply to the occluded marginal branch was recognised at initial diagnosis, this feature is of particular concern. Indeed, in this scenario antegrade coil embolisation, with successful occlusion of the distal marginal branch, may not be sufficient to seal the leakage completely as collateral flow might allow persistent leakage from the ruptured vessel. A contrast injection with the balloon inflated at the marginal branch may provide some insight in this regard. However, a selective contrast injection in the distal left anterior descending coronary artery from a microcatheter may be required to rule out persistent extravasation from this vessel definitively. We have never experienced the unusual unique situation where a ruptured distal vessel continues to leak despite its complete proximal occlusion with embolisation devices. However, in this setting it would appear reasonable to introduce deeply and deploy distally the microcoils starting at the most distal part of the marginal branch in an attempt to occlude the vessel both distally and proximal to the rupture site. Should persistent leakage remain despite this manoeuvre, sequential distal embolisation of the donor collateral vessel from the left anterior descending coronary artery should be attempted.
Conflict of interest statement
The authors have no conflict of interest to declare.
How would I treat?
The invited expert’s opinion
Coronary artery perforation with bleeding into the pericardial space is a rare but feared complication of percutaneous coronary intervention (PCI). Perforation at the lesion site is usually immediately apparent and, in most patients, can be controlled by balloon occlusion, followed by pericardiocentesis and polytetrafluoroethylene (PTFE) covered stent deployment, as necessary. Distal coronary guidewire perforations are less common1,2. Because the hole is usually small, presumably approximately 0.014 inch (0.36 mm) diameter, bleeding may be insidious and either not angiographically apparent or overlooked during the PCI procedure. The patient is often initially asymptomatic, with tamponade gradually developing over the next few hours or up to three days later. Distal perforations are more likely to occur if hydrophilic guidewires are used, and less likely to occlude spontaneously if the patient has received glycoprotein IIb/IIIa inhibitors2.
All cardiac catheterisation laboratories must be equipped to manage coronary and peripheral vascular perforations. A “perforation tray” with everything needed in one place works well. Distal guidewire perforations can be readily treated by occluding the distal vessel. Microcoils are easy to deliver through a microcatheter with an inner lumen able to take a 0.018 inch guidewire, and are radiopaque. We have successfully used microcatheter injection of Gelfoam3 or tris-acryl gelatine microspheres4. Other materials that have been injected to occlude the distal coronary artery include collagen, thrombin, fibrin glue, subcutaneous tissue, polyvinyl alcohol foam, cyanoacrylate glue, and the detached tip of a coronary guidewire. Occlusive agents delivered using a guidewire may be less likely to end up in the wrong place than those injected. In this case we would have located the site of perforation by injecting contrast through a microcatheter, and then delivered a microcoil or small piece of Gelfoam, pushed down with a coronary guidewire.
When hydrophilic or very stiff coronary guidewires are needed during PCI, the likelihood of distal coronary artery perforation can be lessened by fluoroscopic monitoring of the distal tip position during balloon catheter exchanges, and changing to a soft, non-coated wire as soon as possible. Glycoprotein IIb/IIIa inhibitors may not be necessary in a patient with stable angina and an apparently uncomplicated procedure. At least one of the final angiographic views, after wire removal, should profile the distal vascular bed and be of sufficient duration to detect subtle, delayed contrast extravasation. The diagnosis should be considered and urgent echocardiography undertaken in any patient with post-procedural hypotension unresponsive to intravenous fluids, or recurring after fluids are given.
Interventional cardiologists would do well to heed advice given to young sailors over half a century ago: “Perhaps the worst plight of a vessel is to be caught in a gale on a lee shore. In this connection the following ... rules should be observed: 1. Never allow your vessel to be found in such a predicament5.”
Conflict of interest statement
J.A. Ormiston is on the Advisory Boards of, and has received honoraria from, Boston Scientific and Abbott Vascular. M.W.I. Webster has no conflict of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION
Dr. Senguttuvan and colleagues reported a case of coronary perforation grade 3, which is defined by a blood extravasation through a frank perforation (≥1 mm) or spilling into an anatomic cavity1. Although this dreadful complication occurs rarely (0.1% to 3.0%), treatment strategy is challenging and not always effective; in-hospital and long-term clinical outcome is poor and mortality rate is not negligible1-3.
The best treatment strategy of coronary perforations is to prevent them whenever possible. Predictors of coronary perforation are related to2-4: a) clinical factors (female gender, elderly patients, previous PCI or coronary artery bypass graft [CABG]); b) complexity of vessel anatomy and coronary lesions (tortuous vessels, type B2/C lesions, calcified lesions, chronic total occlusion [CTO]); c) anticoagulation/anti-aggregation (glycoprotein IIb/IIIa inhibitors); d) procedural factors (hydrophilic guidewires, higher balloon pressures, larger balloon sizes, rotational atherectomy). Once the perforation has occurred, treatment requires not only immediate actions to seal the perforation, but frequently also measures aimed at haemodynamic stabilisation (i.e., placement of pericardial drain, intra-aortic balloon pump, etc.).
As to this specific case, there is a short occlusion, with a stump and maybe a tiny channel with some distal contrast opacification through retrograde homo-collaterals (as referred by the authors). The age of the occlusion is not known, but angiographically and on the basis of the anamnesis, we would not deem this as a CTO. In a stable, young patient without factors portending to increased platelet reactivity, pre-treatment with dual antiplatelet therapy (i.e., aspirin and 600 mg clopidogrel) and 100 U/kg of unfractionated heparin (UFH) would be administered. There is no need to use IIb/IIIa inhibitors, except in case of suboptimal coronary flow. A supportive guiding catheter (i.e., EBU 4; Medtronic, Minneapolis, MN, USA or Amplatz Left 2, 6 or 7 Fr; Cordis, Johnson & Johnson, Warren, NJ, USA) and a floppy guidewire would be the preferred set-up to start with. There is uncertainty that the inability to cross the lesion is due to the complexity of the stenosis rather than to the poor back-up support (a JL 3.5 [Cordis Corp., Johnson & Johnson, Miami Lakes, FL, USA] was used). In case of failure to cross the lesion, we would switch to a hydrophilic (±tapered) guidewire (i.e., Whisper™ [Abbott Vascular, Santa Clara, CA, USA] or Fielder® XT [ASAHI Intecc, Aichi, Japan]) within a microcatheter (MC) or over-the-wire balloon (OTW). Once the hydrophilic guidewire has crossed the lesion, one important point to prevent distal perforation is to exchange the hydrophilic guidewire over the MC or OTW balloon with a non-hydrophilic guidewire as soon as possible. In addition, it is of paramount importance to hold on firmly to the guidewire (especially the hydrophilic ones) during MC or balloon exchange as well as during contrast injection to prevent brisk distal movement of the tip of the guidewire. A final high quality angiogram is crucial to the prompt diagnosis of distal coronary perforation. In fact, too short an angiogram (like the one shown in Figure 2) is often the reason for a late diagnosis of coronary perforation. With hindsight, a careful look at the final angiogram very often allows the identification of a small distal leakage.
After the diagnosis of pericardial tamponade is made, the patient should immediately be taken back to the cathlab. To achieve haemodynamic stabilisation, pericardial drainage should be performed, but followed right after by a coronary angiogram. There is no point in first trying conservative management, as the cause of pericardial tamponade post PCI should be investigated immediately.
This distal coronary perforation cannot be treated with covered stent implantation. Therefore, prolonged balloon inflation should be attempted first, with partial reversal of UFH (preferably under activated clotting time [ACT] control) (Figure 4). Complete reversal of UFH might, in fact, provoke thrombosis proximal to the inflated balloon or within the guiding catheter, worsening even further the situation with the potential of injecting clots during the control angiogram. In case this manoeuvre is sufficient to achieve haemostasis (50% of the cases), the patient should be closely checked clinically for 24 hours with serial transthoracic echocardiogram in order to detect possible recurrence.
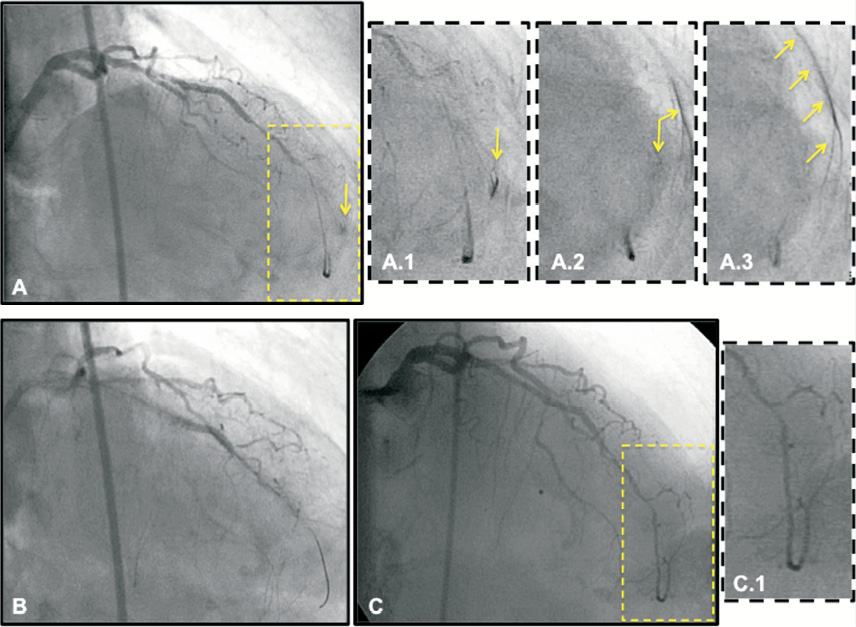
Figure 4. This is an 82-year-old female patient treated with PCI of mid-left anterior descending artery (LAD) CTO. The procedure was technically demanding and required the use of hydrophilic guidewires and specialty catheters (Tornus [Abbott Vascular, Santa Clara, CA, USA], Corsair [ASAHI Intecc, Aichi, Japan]) to cross the occlusion. (A) After guidewire removal, a final angiogram was performed that showed an acceptable result with regard to the stented segment and diffusely diseased distal LAD segment. After careful reading of the angiogram, a small grade 3 perforation (A.1 to A.3) was noticed at the level of a tiny distal diagonal branch (most likely perforated during the procedure by the guidewire); (B) Prolonged balloon inflation (2.0×20 mm) was performed in the distal LAD (selective wiring of the relevant branch was not possible) and protamine sulfate was administered to neutralise half of the heparin administered; (C) Control angiogram after 30 min shows effective haemostasis and sealing of the distal coronary rupture. The patient was transferred to the coronary care unit (CCU) for 24 hours; transthoracic echocardiogram performed three and 12 hours later did not show any significant pericardial effusion. The patient was discharged uneventfully.
If balloon inflation is ineffective, haemostasis should be attempted with distal embolisation of thrombogenic compounds. Successful sealing of distal coronary perforations has been reported using injections, through MC or OTW balloons, of thrombin, fibrin glue, collagen from Angio-Seal™ (St. Jude Medical, St. Paul, MN, USA) closure device, microcoils, gel foam, polyvinyl alcohol and organised thrombus from the same patient6-11. If these measures are not effective, then there is no alternative but surgical repair.
Conflict of interest statement
The authors have no conflict of interest to declare.
How did we treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Balloon tamponade of the distal vessel was performed with a 2×15 balloon for 30 minutes, which resulted in transient improvement. Once the balloon was deflated, the pericardial effusion started re-accumulating rapidly. Hence, it was decided to seal the leak. The options considered included placement of covered stent or injection of microcoils, thrombin, collagen, gel, polyvinyl alcohol, subcutaneous tissue or glue1-10. A covered stent was not considered due to the small diameter of the involved artery, whilst microcoils may not have been successful as the patient was on tirofiban and absolute sealing was desired due to persistent, refractory leak. Hence, we proceeded to seal the perforation with cyanoacrylate glue.
A 0.014” Tracker® microcatheter (Boston Scientific, Natick, MA, USA) was placed as distal as possible over a 0.014” stabiliser (Cordis Corporation, Johnson & Johnson, Miami, FL, USA) exchange length wire to the point proximal to the perforation (Figure 5). The entire catheter system was flushed well with 5% dextrose solution to avoid the contact of glue with blood. A mixture of 0.25 ml of cyanoacrylate glue (B. Braun Biosurgicals, Ann Arbor, MI, USA) mixed with 0.25 ml of lipoidol was injected rapidly and carefully to avoid the proximal spillover of the glue. The catheter system was again flushed with 5% dextrose solution. Pericardial collection stopped immediately and the haemodynamics improved. The angiogram revealed no leak (Figure 6).
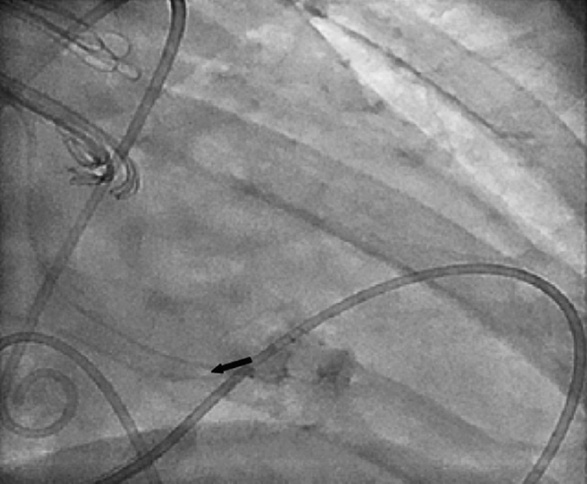
Figure 5. LCA angiogram showing the placement of the microcatheter proximal to the site of perforation through a JL guiding catheter.
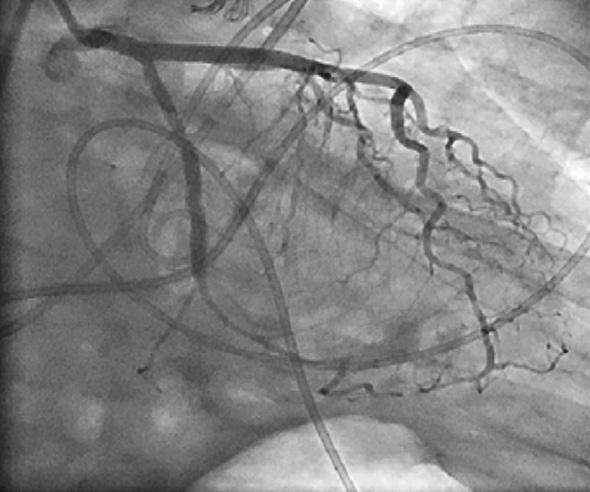
Figure 6. LCA angiogram in right anterior oblique caudal view showing complete sealing of contrast leak following injection of cyanoacrylate glue.
N-butyl cyanoacrylate glue, commonly known as super glue, is frequently used in the management of bleeding peripheral vessels and bleeding varices. The glue has previously been used to seal left ventricular free wall rupture11, to seal cardiac perforation during placement of pacemaker leads and balloon mitral valvotomy12, and septal perforator ablation in hypertrophic cardiomyopathy13. It has been used successfully to seal a coronary perforation during PCI in the right coronary artery. The hydroxyl ions in the super glue immediately polymerise once it comes into contact with the Na+ ions. Hence, a sandwich technique, in which the entire catheter is flushed with non-ionic dextrose solution before and after instillation of the glue, should be done. The glue should be placed as close to the injured portion of the vessel as possible in order to avoid the spillover of glue, which can be a catastrophic event. The glue should be placed as rapidly as possible so as to avoid the contact of glue with the blood inside the catheter. Since the area supplied by the distal branch was small, it did not cause any problem to us immediately. The patient was discharged in a stable condition two days later on clopidogrel, aspirin, beta-blockers, statins and ramipril.
Four weeks later, the patient presented with fever and chest pain suggestive of pericarditis. Echocardiography showed mild pericardial effusion. The possibilities included infectious pericarditis, Dressler’s syndrome, and glue-induced pericarditis. Intravenous antibiotics were started though the clinical picture was not suggestive of pyogenic pericarditis. Low dose steroid (20 mg prednisolone once daily) was started, following which the clinical improvement was dramatic. When the steroids were tapered off four weeks later, there was a recurrence of symptoms. A trial of colchicine (0.5 mg two times daily) was given with which the patient remained asymptomatic and free of steroids for four months.
The recurrent pericarditis was the most unusual complication encountered in our patient. The glue is an irritant and has been used to produce adhesions in critical cardiac surgery. In our patient, only a small amount of glue was put into the coronary artery. The glue must have spilled into the pericardial space to produce a subtherapeutic effect leading to pericarditis. To the best of our knowledge, such glue-induced pericarditis is not reported in the literature. Fortunately, the glue-induced recurrent pericarditis responded to steroids and colchicine.
Conflict of interest statement
The authors have no conflict of interest to declare.
Online data supplement
Moving Image 1. Left coronary artery (LCA) angiogram in right anterior oblique view with caudal angulation showing total occlusion of the left circumflex artery retrograde filling of a good-sized obtuse marginal artery.
Moving Image 2. Coronary angiogram showing successfully deployed stent with TIMI 3 flow attained at the end of the procedure.
Moving Image 3. Coronary check angiogram showing extravasation of contrast from the terminal branch of obtuse marginal artery and a well deployed stent across the lesion.
Moving image 4. Coronary angiogram showing passage of a stabiliser wire towards portion, proximal to the distal coronary perforation.
Moving image 5. LCA angiogram showing the placement of the microcatheter kept proximal to the site of perforation through which the glue is injected.
Moving image 6. LCA angiogram in right anterior oblique caudal view showing complete sealing of contrast leak following injection of cyanoacrylate glue.

