
CASE SUMMARY
BACKGROUND: A 90-year-old lady with a degenerated 23 mm Sorin “Solo” stentless aortic bioprosthesis was referred to our outpatient clinic. She was heavily symptomatic for dyspnoea (NYHA Class IV) and, due to her advanced age, she was referred to screening for TAVI. Pre-procedural evaluation confirmed the presence of a degenerated aortic bioprosthesis, with severe aortic regurgitation and moderate aortic stenosis.
INVESTIGATION: Clinical assessment, echocardiography, cardiac catheterisation including coronary angiography and aortography, cardiac CT.
DIAGNOSIS: Structural valve degeneration of the aortic bioprosthesis with complete detachment of the left coronary cusp, determining severe aortic regurgitation and moderate aortic stenosis. Moderate mitral regurgitation. Moderate tricuspid regurgitation. Pulmonary hypertension. High risk of coronary occlusion during valve-in-valve implantation (as estimated on CT).
MANAGEMENT: Redo aortic valve replacement.
KEYWORDS: aortic valve replacement, redo surgery, TAVI, valve-in-valve implantation
PRESENTATION OF THE CASE
A 90-year-old lady with resting dyspnoea was addressed to our outpatient clinic for evaluation. In 2005 she had undergone aortic valve replacement with a 23 mm stentless Sorin Freedom “Solo” aortic bioprosthesis (Sorin [now LivaNova], Saluggia, Italy). She had a 60% stenosis of the left carotid artery and chronic obstructive pulmonary disease (COPD; asthma). Transthoracic echocardiography confirmed the presence of marked structural deterioration of the aortic bioprosthesis, with almost complete detachment of the left coronary cusp and severe aortic regurgitation. The logistic EuroSCORE I was 46.8%, due to the following: female gender, advanced age, extracardiac arteriopathy, previous cardiac surgery, and “other than isolated CABG”. The STS risk score for mortality was 6.76%. The STS risk score for morbidity and mortality was 31.8%. Despite her advanced age and the presence of severe symptoms, the patient was extremely active, was living alone and taking care of a disabled son.
The patient was brought to the attention of our institution’s TAVI team. All the team members had the perception that she deserved to be treated, but the risk of a conventional surgical procedure was judged significant. On the other hand, it is generally recognised that transcatheter valve-in-valve implantation into a degenerated Sorin “Solo” valve is associated with a significant risk of coronary ostial occlusion1-3, due to the prosthesis design and to the surgical implantation technique. In fact, this prosthesis has to be sutured to the wall of the sinus of Valsalva, well above the native aortic valve annulus and close to the origin of the coronary arteries4,5. To understand the patient’s anatomy better, and to define the potential advantages and drawbacks of all the available strategies, a CT scan for TAVI planning was requested. The exam demonstrated the high position of the degenerated prosthesis in the aortic root, and its close relationships with the coronary artery ostia. Of note, as originally prescribed by the manufacturer, the prosthesis had been implanted well above the level of the native aortic valve annulus, determining the formation of a circumferential “cul-de-sac” between the prosthesis insertion and the virtual basal ring (Figure 1).
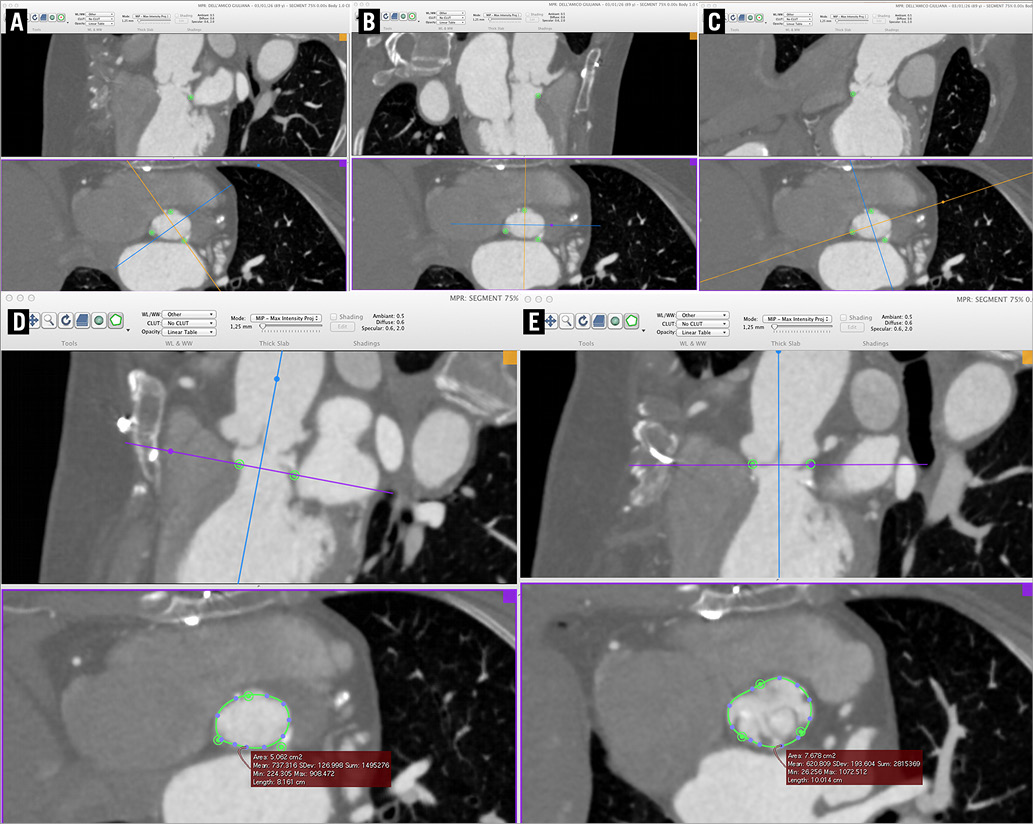
Figure 1. Pre-procedural CT scan. The level of the true aortic virtual basal ring is well below the prosthetic cusps, and there is a “cul-de-sac” between the original aortic annulus and the insertion of the Solo valve. The nadir of the three sinuses is identified and marked as usual: left coronary (A); right coronary (B); acoronary (C). Note the close proximity of the prosthesis leaflets’ insertion to the coronary ostia. D) Area of the true virtual basal ring. E) Area of the “prosthetic” virtual basal ring.
Should this lady be referred for optimal medical therapy? Or should the risk of conventional surgery be accepted, considering the patient’s strong self-reliance and the good performance status experienced until recently? Or, finally, should transcatheter therapy be attempted?
How would I treat?
THE INVITED EXPERT’S OPINION

Coronary obstruction after transcatheter aortic valve implantation (TAVI) inside a failed surgical bioprosthesis (valve-in-valve [ViV]) is a life-threatening complication2,6,7. Occasionally, as in the case described, the risk of this serious adverse event is especially high. Since ViV outcomes in patients with an increased risk of coronary obstruction are suboptimal, alternative strategies should be sought8. Repeat cardiac surgery, in which the failing surgical valve is removed and a new valve is implanted, would be unusual in this case of a 90-year-old lady with numerous comorbidities. For this patient, medical treatment should be considered. However, exceedingly poor prognosis will be associated with conservative treatment in this highly symptomatic patient, as the patient has a recent history of being “extremely active”. Therefore, the risks associated with ViV seem acceptable.
ViV is increasingly being performed in patients at increased risk of coronary obstruction9-11. Even though most ViV procedures are currently performed under a minimalist approach, in high-risk cases, such as the case described, general anaesthesia and transoesophageal echocardiogram (TEE) surveillance should be considered. This would enable rapid diagnosis of coronary obstruction, such as new wall motion abnormalities, while excluding other aetiologies for haemodynamic instability, and would allow rapid patient stabilisation in case a complication occurs. Selecting the type of transcatheter heart valve (THV) is challenging. Several devices are retrievable and a few can even be retrieved after full deployment. Full retrievability could be of significant benefit in cases at risk of coronary occlusion. If a complication occurs, then the THV device could be promptly removed and the coronary flow would resume. However, unfortunately coronary obstruction may also occur hours or even days after the ViV; therefore, not having a complication immediately after implantation does not guarantee having normal flow later. Another strategy would be to use a device with a leaflet clipping mechanism (i.e., JenaValve™; JenaValve Technology, Inc., Munich, Germany). This may enable less lateral displacement of the surgical valve leaflets and, theoretically, would decrease the risk of coronary obstruction. Another advantage of the JenaValve, in this particular case, lies in its ability to anchor in isolated aortic regurgitation pathology, with only mild leaflet calcification, as in the described case. Irrespective of the type of THV to be utilised, there should be coronary protection during all ViV cases at risk of coronary occlusion, using a wire and an undeployed stent that are placed in the coronary tree before THV deployment11. This technique may enable implanting the coronary stent rapidly, if an obstruction occurs. Attempting to wire the coronaries after occlusion is often unsuccessful.
Therefore, my approach for treating the described challenging high-risk patient would be to implant a JenaValve under general anaesthesia and TEE, while having active coronary protection, which includes a wire and stent. Potential limitations of this strategy include the fact that JenaValve ViV may also result in coronary obstruction and therefore the risk for this ominous complication will not be eliminated (Figure 2). In addition, JenaValve ViV is associated with relatively high post-procedural gradients in ViV cases. However, these were described mainly when implanting in small and intermediate stented surgical valves (Figure 3).
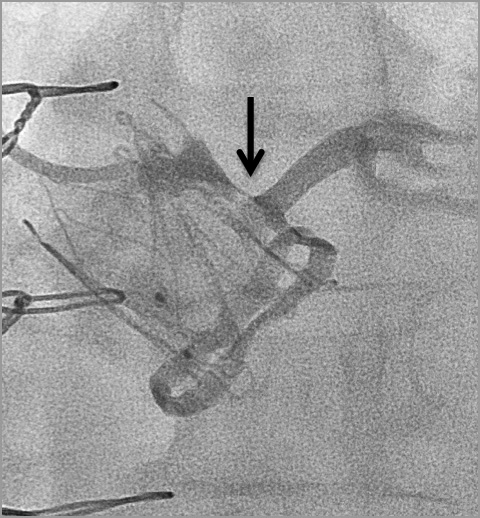
Figure 2. Coronary obstruction following JenaValve aortic ViV in a failed Mitroflow (Sorin) bioprosthesis.
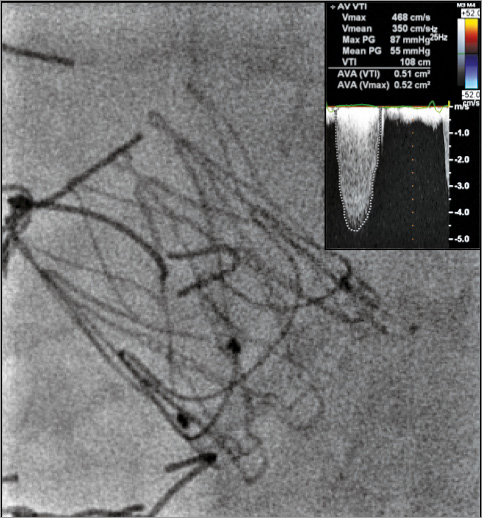
Figure 3. Severely elevated post-procedural gradient after JenaValve ViV inside a failed PERIMOUNT (Edwards Lifesciences) #23 with mean gradient of 55 mmHg.
In general, it seems that meticulous procedural planning and postoperative evaluation may allow successful device implantation and optimisation of clinical outcomes in high-risk patients with failed bioprosthetic valves12.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION

The life expectancy of patients who have received a tissue valve in their seventies and eighties has steadily increased over the last two decades. This results in clinical scenarios like the one described above: the patient has reached 90 years of age and presents with severe symptoms due to a degenerated bioprosthesis.
Ten years ago, only two options could have been proposed to these patients: palliative medicamentous treatment or conventional surgical redo procedure. At that time, the perioperative risk was not estimated with scoring systems. Not infrequently, surgeons made decisions using “weak” criteria or even intuition. The questions were: is surgery a realistic option and is it wise to offer it?
Today, the decision may be facilitated by the calculation of different scoring systems. On the other hand, this may be complicated by the fact that different “actors” want to catch the patient. In fact, every risk scoring system massively overestimates the effective mortality and morbidity when the procedure is performed in an expert centre with a large operative volume and a high case load of aortic valve surgery. In our institution, for instance, mortality in isolated AVR has approached 0.5% in recent years (data validated by the IQM). Our experience with very old patients has not been that large in recent years, but, out of 181 patients over 80 years of age and with STS risk factors of between 2% and 7%, overall mortality has been less than 1.5%. Even isolated redo AVR for degenerated tissue valves can be performed with a mortality rate of less than 2%, independently of the age of the patients.
This information has been confirmed recently with excellent results obtained following surgical aortic valve replacement in elderly patients: out of 3,735 patients (age 80 or older) operated on in one centre and reported to the STS database, 61 patients needed a redo procedure because of tissue valve degeneration13. The average age of the patients was 83±2 years, 77% were male, and 75% had undergone an isolated coronary artery bypass graft as the previous cardiac procedure. A stented valve was implanted in 61% of patients and a stentless valve in 39%. Perioperative mortality was 1.6% (one of 61).
This underlines the fact that scoring systems unfortunately reflect only the average quality of surgery over multiple institutions. Therefore, the individual performance of a particular institution may be better or worse than average and this should be given more consideration.
Technical aspects
In the early experience, most valve-in-valve (ViV) TAVI procedures were performed using a transapical approach, mainly for technical reasons and, in the majority of cases, ViV procedures were performed in stented bioprostheses. Thus, the rigid design of a stented valve makes placement easier, does not usually induce a major interference of the valve leaflets with the coronary ostias, and guarantees that the transcatheter valve is optimally anchored.
Kelpis et al reported a transapical ViV TAVI procedure for the treatment of a degenerated Toronto SPV® stentless porcine valve (St. Jude Medical, St. Paul, MN, USA) using the Edwards SAPIEN™ bioprosthesis (Edwards Lifesciences, Irvine, CA, USA)14, while other authors have described successful TAVI in another model of stentless bioprosthesis15 (Shelhigh NR2000; Shelhigh, Inc., Millburn, NJ, USA).
ViV TAVI procedures may represent special challenges, mainly regarding the optimal choice of the most appropriate device, especially when a stentless valve had previously been implanted16. The absence of stents and radiopaque markers makes visualisation of the tissue valve and of the aortic annulus more difficult; therefore, proper positioning of the transcatheter valve is more challenging. Among others, two specific points have to be discussed when a ViV TAVI is performed in a stentless bioprosthesis:
1. The lack of strong support (through the sewing cuff, such as in a stented valve) means that stable fixation of the transcatheter valve is more difficult. This may result in a higher risk of valve displacement.
2. In case of leaflet tear of the stentless bioprosthesis, the risk that such a partially “floating” leaflet obstructs a coronary ostium should be considered.
Coronary obstruction is usually caused by a leaflet of the bioprosthesis that is pushed towards a coronary ostium. This mechanism has to be considered in case of a degenerative tear which makes the leaflet more mobile in the aortic root5. This may be particularly true for a stentless valve such as the Solo valve that fulfils at least three criteria:
– it is a stentless valve,
– the profile of the leaflets is high,
– it is implanted in a supra-annular position.
Proposal for the patient
Surgery is of course possible in this patient and the probability of adverse outcome in an expert centre is less than 2%: this would be my preferred approach. Vola et al reported the use of a quick sutureless valve implantation in a patient with a degenerated Solo valve: this could be of interest to offer a simplified and quick surgical procedure 17, but the patient may prefer a less invasive procedure. Despite this excellent perspective, it is obvious that most probably a TAVI procedure will be proposed to this patient. In this case, it is important to understand the specific fixation of the Solo stentless valve with one suture just above the annulus level and the fact that aortic regurgitation is due to a torn leaflet which may be floating close to the corresponding coronary ostium. This means that a device which may be able to grasp the leaflets rather than to push them in the direction of the coronary artery should be used. One possibility may be the JenaValve that may fixate at the cusps of the stentless valve through the unique clipping mechanism. Other TAVI devices that could easily be retrieved (Evolut™ R [Medtronic, Minneapolis, MN, USA], Portico™ [St. Jude Medical], Lotus™ [Boston Scientific, Marlborough, MA, USA]) in case coronary occlusion occurs may be advantageous. If such a complication occurs, it may be treated either by PCI or by emergency surgical intervention following peripheral cannulation in case of haemodynamic collapse.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Our routine approach for high-risk patients with malfunctioning bioprostheses, e.g., transcatheter valve-in-valve implantation with a balloon-expandable valve, was judged not feasible in this case, as the detailed analysis of the CT images left little doubt about the risk of coronary ostial occlusion, which was extremely high. A second option was valve-in-valve (ViV) implantation of a fully retrievable transcatheter device with prophylactic wiring of the coronary arteries for protection. However, this alternative was also discarded. In fact, the deployment of a transcatheter valve could have added significant damage to the malfunctioning prosthesis, and removing the device to free an occluded coronary artery could have resulted in massive aortic regurgitation and non-sustainable haemodynamic deterioration.
Transapical valve-in-valve implantation with the JenaValve has been successfully used in patients with degenerated “Solo” prostheses18. This self-expanding nitinol valve has a peculiar anchoring mechanism that resembles a trefoil paper clip, and relies on active clip fixation of the native aortic valve or degenerated stentless prosthesis leaflets, preventing their displacement towards the coronary ostia19. In the present patient, however, we felt that using a JenaValve would have been a gamble. In fact, one of the three valve leaflets was almost completely detached from its insertion line. Moreover, the sizing of the transcatheter valve would have been complex, as there was a significant discrepancy between the size of the true aortic valve virtual basal ring and the prosthetic neoannulus where, due to its anchoring mechanism, the JenaValve would have been seated (Figure 1D, Figure 1E).
Albeit more traumatic, and despite the high calculated risk scores, conventional surgery was considered the safest and the easiest option for this patient. The operation was performed via a full resternotomy. At surgical inspection, the echocardiographic diagnosis was confirmed: the left coronary cusp was almost completely detached from the sinus of Valsalva wall, and the non-coronary cusp was calcific and hypomobile. After the removal of the prosthetic cusps, the cul-de-sac between the true aortic annulus and the prosthetic neoannulus became evident (Figure 4). To avoid the removal of the pericardial sewing cuff, which could have resulted in significant damage to the aortic wall, we decided to implant a sutureless prosthesis. Three guiding sutures were passed at the nadir of the cusps, and a size L Perceval™ S valve (Sorin) was delivered at the level of the native aortic annulus and released. After checking its correct positioning, the prosthesis was expanded with a dedicated balloon catheter. The prosthesis stent levelled the prosthetic neoannulus, leaving the coronary ostia wide open. The aortic cross-clamp time was 38 minutes, and the cardiopulmonary bypass (CPB) time was 55 minutes. The patient was extubated after six hours and discharged to the general ward on postoperative day one. The subsequent course was uneventful, and she was discharged on postoperative day six. The pre-discharge echocardiography showed a well-functioning prosthesis, with a mean gradient of 11 mmHg and no residual regurgitation. At the one-month follow-up visit, the patient was asymptomatic for dyspnoea, and in good general condition. The mean transprosthetic gradient across the aortic prosthesis was 8 mmHg, with no AR.
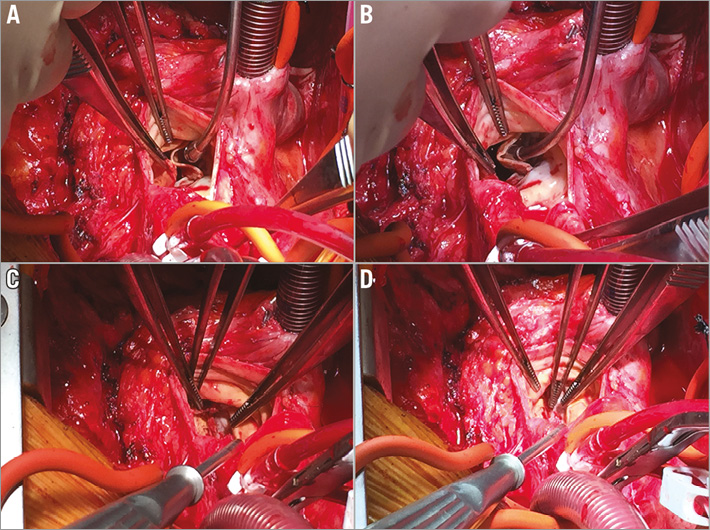
Figure 4. Intraoperative view. A) & B) Intraoperative inspection confirms the presence of significant structural valve deterioration, with almost complete detachment of the left coronary cusp from the aorta. C) The pericardial leaflets of the Solo valve have been resected leaving the sewing cuff in place. D) The level of the true virtual basal ring lies below the prosthesis insertion line.
The Sorin “Solo” stentless aortic bioprosthesis was introduced in 2004 as an alternative to conventional aortic bioprostheses. Due to its peculiar design, it is a valid option for patients with small aortic annuli and at increased risk of prosthesis-patient mismatch20. Patients with degenerated Sorin “Solo” bioprostheses, however, may be at increased risk of complications both during ViV implantation (coronary occlusion, prosthesis malposition1) and during conventional surgery (damage to the aortic wall during the removal of the “Solo” prosthesis). For this reason, careful planning based on preoperative imaging and in-depth evaluation of the individual patient in the setting of a Heart Team is essential. In the present patient, the risk of coronary complications was extremely high. On the other hand, we felt that the risk of an open heart procedure was overestimated by the EuroSCORE and STS score, since the patient was not as fragile as her age could have suggested. Moreover, the use of a sutureless prosthesis reduced significantly the cross-clamp time and CPB time, and avoided the removal of the prosthesis sewing cuff, a manoeuvre that carries a significant risk of damaging the surrounding cardiac structures.
In conclusion, we believe that open heart surgery is an excellent option for some high-risk patients with degenerated aortic bioprostheses. Careful evaluation of the patient’s anatomy is essential, and a strongly multidisciplinary, Heart Team-based approach is the key to success in this complex situation.
Conflict of interest statement
A.G. Cerillo and M. Solinas have received speaker honoraria from LivaNova. The other authors have no conflicts of interest to declare.

