
Abstract
Aims: The purpose of this study was to assess the incidence and predictors of graft failure prior to discharge. Multi-slice computed tomography has the ability to evaluate graft patency in a non-invasive way.
Methods and results: Of 145 consecutive patients who were screened, 73 were included in the study (78% male, mean age 65 years). A total of 206 grafts were analysed (2.8±0.9 grafts/patient). Of the 206 grafts, 126 were venous, 72 were left internal mammary, five were right internal mammary and three were radial grafts. We evaluated 100% of proximal anastomoses sites and 92% (190/206) of the distal anastomoses. We identified five patients (6.8%) who had at least one occluded graft. A total of seven out of 206 (3.4%) grafts were occluded. Independent predictors of successful graft outcome were left anterior descending artery as a recipient artery, good distal run-off as assessed by a surgeon and vessel size larger than 2 mm.
Conclusions: The results demonstrate that the in-hospital acute graft failure rate is 3.4% (6.8% of patients). Multi-slice computed tomography is a robust technique to assess novel therapies to reduce the rate of graft attrition further, and might be clinically useful in patients with persistent or early recurrence of symptoms after CABG.
Introduction
Coronary artery bypass graft (CABG) surgery, using venous or arterial conduits, is an established treatment for obstructive coronary disease1. Historical data, based on serial invasive coronary angiograms, suggest that up to 15% of venous grafts become occluded within twelve months of the procedure; limited data suggest that a substantial proportion of such occlusions occur much earlier2,3. Previous studies evaluating the incidence of graft occlusion utilised catheter-based coronary angiography to assess the patency of bypasses4,5. However, such an approach has the inherent tendency of restricting the study population only to patients with clinical conditions for undergoing an invasive procedure, or to those with clinical ischaemic events. Multi-slice spiral computed tomography (MSCT) is a relatively new technique which can evaluate coronary graft patency in a non-invasive fashion and provide a means to gain an insight into the early fate of bypass grafts in the current era. The relative immunity of bypass grafts to motion artefact, coupled with their size, makes them ideal structures for evaluation by MSCT. Even the earlier generations of scanners had high sensitivity and specificity for the detection of stenosis in bypass grafts. This has further improved with the recent generation of 64- and 128-slice scanners6-12.
To our knowledge, limited data13 exist on the incidence and the determinants of graft occlusion in the early postoperative period, assessed in a non-invasive way with the old generation of 16-slice scanners. Additionally, the improved diagnostic accuracy of 64-slice MSCT scanners for evaluating midterm and long-term graft patency has been demonstrated9,14,15. We therefore undertook a prospective study with a dual-source MSCT scanner to assess the occurrence of in-hospital graft occlusion in patients undergoing CABG.
Materials and methods
STUDY DESIGN AND POPULATION
Two highly experienced surgeons, familiar with both off-pump and on-pump techniques, participated in the study. Exclusion criteria were irregular heartbeat, contrast allergy, renal insufficiency, and lung disease which precluded breath hold. All patients gave written informed consent and the protocol was approved by our institutional review board. Out of 145 consecutive bypass patients with no concomitant valve disease (the surgical bypass being performed by two surgical teams), 73 were included in the study (63 patients by surgical team A and 10 by surgical team B, with 173 and 33 grafts carried out, respectively). The main reasons for excluding patients were mild renal impairment (40) or atrial fibrillation (23) postoperatively. All of the patients were operated on immediately after the diagnostic catheterisation. Consequently, in patients with postoperative renal impairment, MSCT was not carried out since, potentially, we could have worsened the renal function. Evaluation of grafts might be feasible even when irregular heartbeat is present. However, we did not scan patients with postoperative atrial fibrillation since in most studies, even the recent ones, these patients are commonly excluded. MSCT was carried out before discharge, generally on the fifth or sixth day after surgery. Details of the type of graft, the site of the distal anastomosis, and other relevant parameters were prospectively collected, on a prospectively defined data collection form, at the time of surgery.
SCAN PROTOCOL AND DATA ANALYSIS
MSCT was performed with 2×32 detectors with double z sampling resulting in simultaneous acquisition of 2×64 overlapping slices (SOMATOM Definition; Siemens, Erlangen, Germany). Tube rotation time was 330 ms, resulting in an effective temporal resolution of 83 ms. Scan parameters were: detector collimation, 64×0.6 mm, tube voltage 120 kV, and tube current between 800 and 900 mAs (depending on the patient’s size). Dose modulation (Siemens “CARE Dose”) was always activated. Effective radiation dose varied from 10.7 to 23.4 mSv with a median of 13.3 mSv. If arterial grafts were present, the scan range was cranially extended to include the origin of the mammary arteries. The table speed was adjusted to the patient’s heart rate automatically. The mean scan time was 15.4+2.3 s (range 11.9-22.4 s). All patients were already taking beta-blocking drugs. Mean heart rate was 72 beats per minute (bpm). If the pre-scan heart rate was >80 bpm, atenolol (1 mg) was administered intravenously. Additionally, all patients received 1 mg isosorbide dinitrate sublingually five minutes before scanning. A bolus of 100 mL of Iopamiro 370 mg/mL (Iopamiro®; Bracco, Milan, Italy) was intravenously injected at a rate of 5 mL/s. Bolus tracking, i.e., monitoring of contrast enhancement in the aortic root during contrast injection, was used to synchronise data acquisition with maximum contrast enhancement. The scanning protocol was retrospective, using the 75% pulsing window unless movement artefacts were detected. Generally, the examination, including patient preparation and image reconstruction, required between 30 and 40 minutes. All examinations were performed without complications.
The reconstructed image data were transferred to an off-line work station (LEONARDO; Siemens Medical Solutions) for post-processing. Images were reconstructed with 0.75 mm slice thickness, usually using the 75% pulsing window. When the image quality was poor, reconstruction was performed for 70%, 80%, 35% or other pulsing windows. MSCT data were analysed by two independent observers who were given details of the number of grafts performed. They were not aware of the type (arterial or venous) of graft or the site of the distal anastomoses. They classified each bypass graft as patent or occluded.
Statistical analysis
Continuous variables were presented as mean±standard deviation (SD) and categorical variables were presented as counts and percentages. An exploratory analysis using logistic regression models was performed to identify univariate predictors of graft occlusion among all clinical, angiographic, procedural and MSCT characteristics. As the number of occlusions was relatively small, the analysis was restricted to the identification of univariate predictors, and no multivariate model was constructed to assess the multivariate value of each predictor.
Results
The patients we studied had a high incidence of known cardiovascular risk factors. Approximately half of the population had diabetes, 41% were current smokers, and 46% had suffered a previous myocardial infarction. The vast majority had multivessel disease (Table 1). Just over half of the surgical procedures (53%) were performed off-pump.
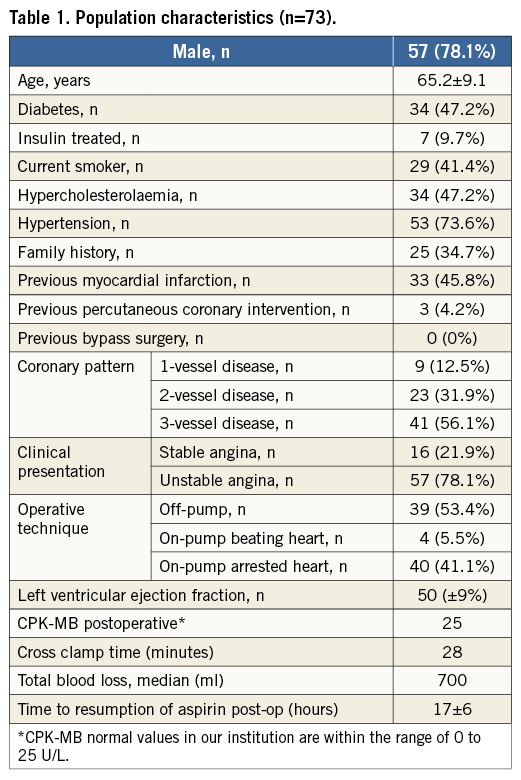
A total of 206 grafts were analysed (2.8±0.9 grafts per patient). All grafts were anastomosed to a single coronary artery. Of the 206 grafts, 126 were venous, 72 were left and five right internal mammary, and three were radial grafts (Table 2). In most cases, distal run-off was judged to be adequate (evaluated during the operation by Doppler flow). However, as described in the operation records, one third of grafted vessels had a moderate or poor distal native bed, 40% were smaller than 2 mm, and approximately half had moderate or severe calcification (Table 2).
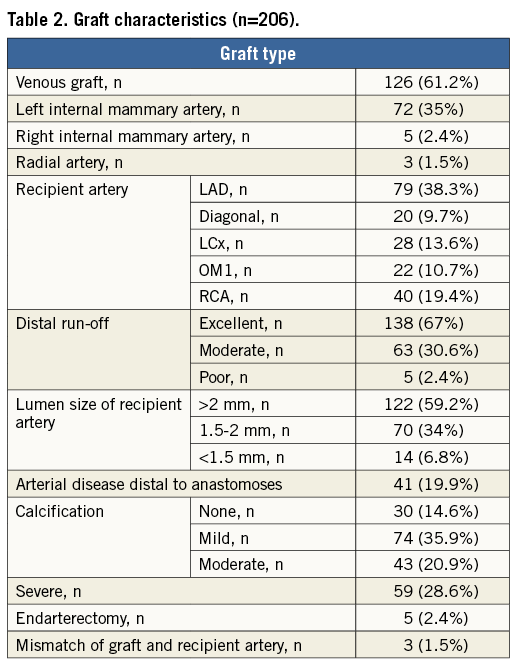
We identified five patients (6.8%) who had at least one occluded graft. A total of seven (3.4%) of 206 grafts were occluded (a detailed description is provided in Table 3). The occlusion, in all cases, was located at the site of the aortic anastomosis, on MSCT. Two patients (patients 9 and 10) had two grafts occluded, as illustrated in Figure 1 (patient 10). The majority of the occlusions (four, 80%) were silent with no electrocardiographic changes and no elevation of cardiac-specific biomarkers. The only patient with elevated cardiac biomarkers (patient 26) had a non-Q-wave myocardial infarction. This patient had undergone extensive endarterectomy.
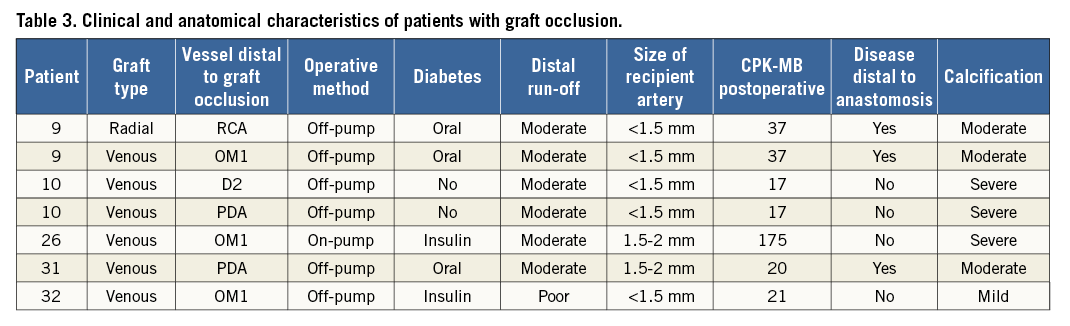
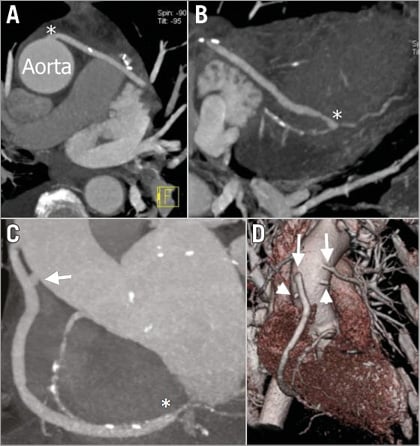
Figure 1. Patient 10. A) & B) Maximum intensity projections of a saphenous vein graft to the left circumflex artery. An asterisk in panel A indicates the site of the anastomosis with the aorta and in panel B indicates the site of the anastomosis with the native circumflex. C) The arrow indicates the occluded graft to the posterior descending artery and the asterisk indicates the site of the anastomosis with the right coronary artery. D) A three-dimensional volume-rendering reconstruction; arrowheads indicate occluded vein grafts to the posterior descending and second diagonal branches. Arrows indicate patent vein grafts to the right and left circumflex coronary arteries.
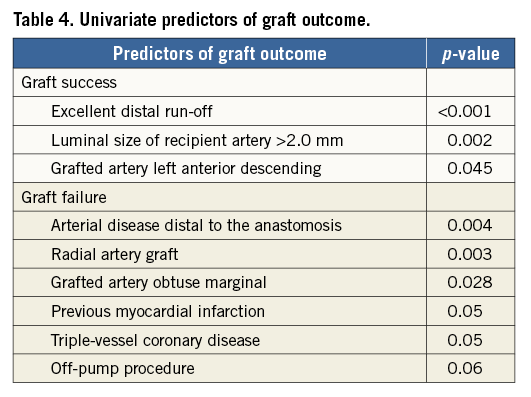
Independent predictors of successful graft outcome were left anterior descending artery as a recipient artery, good distal run-off as assessed by a surgeon, and vessel size larger than 2 mm. Predictors of graft outcomes are presented in Table 4. The distal anastamosis and the quality of the distal run-off were evaluable in 190 (92%) of the 206 distal anastomoses. Calcification, artefacts, or small vessel size (singly or in combination) were responsible for the inability to evaluate these parameters by MSCT.
Discussion
The major finding of our study was that in-hospital graft occlusion occurred in 6.8% of patients and 3.4% of grafts. This compares favourably with the rate of graft occlusion in the early postoperative period in historical studies and suggests that advances in medical management, such as the continuation of aspirin in the perioperative period and the widespread use of statins, may have impacted on the rate of early graft attrition.
In all patients with graft occlusion, the distal native vessel was visible with MSCT. Subcritical lesions with competitive antegrade flow may lead to early graft occlusion. However, in-hospital graft occlusion is mainly attributed to intraoperative reasons such as technique and proper harvesting of the graft. All patients who had graft occlusion had surgery in the setting of a recent myocardial infarction. The documented increased inflammatory response and prothrombotic milieu in patients with recent infarction are plausible explanations for the observed increase in graft occlusion in this setting. The use of the radial artery as a conduit was related to graft failure, as previously reported16. Our results are in line with a recent study showing in-hospital 50% radial artery graft patency13. We therefore believe that total arterial revascularisation by using the radial artery should be performed selectively. Off-pump surgery was performed in 36 patients. There was a very high (25%) rate of acute graft occlusion in the subset (n=16) who had three-vessel disease. This may reflect the fact that these patients had off-pump surgery because it was a less invasive technique in a higher-risk patient subset. However, there was a trend for off-pump surgery to reach significance as a predictor of graft failure. This trend is consistent with a recent study in a large cohort of patients17. On the other hand, one study (with a single surgeon) demonstrated similar results between on- and off-pump surgery18. A recent report found that prolonged pump time was a predictor of graft failure13. In our series, on-pump time was not associated with graft failure. This may reflect different operative techniques, pump protocols and individual experience. Interestingly, 80% of patients with graft occlusion had diabetes. The BARI study suggested that the presence of diabetes had no influence on graft patency over a four-year period19. However, patients with diabetes have an increased propensity for thrombosis and smaller vessels, with more diffuse disease, compared to controls. These factors might potentially predispose to an increased risk of acute graft failure. Graft failure is related to three different time-dependent mechanisms. Thrombosis is the principal cause of acute graft failure, whereas fibrointimal hyperplasia and the development of atherosclerosis in the graft are the major factors in late graft failure20. In the present study, as reported in Table 3, moderate to severe calcification, small diameter, and impaired distal flow related to distal disease were factors that predisposed to graft occlusion.
While acute stent occlusion has serious clinical consequences, graft occlusion did not cause symptoms in any patient in this study. This suggests that perfusion from the native vessels, or via collaterals, was adequate to maintain myocardial perfusion, or that the grafted territory was already infarcted. This does not imply that unnecessary grafts were performed. The decision to graft an individual vessel at surgery is based on visual assessment of the degree of stenosis on angiography in conjunction with perioperative assessment of the quality of the distal vascular bed. Visual assessment of angiograms has well-known limitations, such as the planar silhouette of the lumen, computer-assisted techniques that rely on the adjacent “normal” wall, motion artefacts, low-resolution images, inability to visualise small vessels and inability to evaluate the vessel’s cross-sectional area. Moreover, the adequacy of collateral perfusion requires techniques that would be inappropriate in patients with diffuse multivessel disease.
Of the five patients with acute graft occlusion, four had grafts whose targets were distally located small-diameter vessels. Given the small territory involved and the lack of symptoms, intervention was not considered. The other patient, whose graft supplied a large dominant right coronary artery, was offered intervention but refused. Although the principal aim of the study was to evaluate graft occlusion before discharge, we obtained follow-up information (by telephone) regarding clinical status at a mean of 12 months post CABG in 71 patients. All were alive and asymptomatic.
The introduction of dual-source scanners in coronary imaging has provided higher temporal resolution and reduced radiation doses, especially at higher heart rates21,22. Conversely, image quality is still hampered by heart rate variability and calcification23,24. MSCT has demonstrated high sensitivity and specificity for the detection of occlusion within grafts, even with 16-slice scanners25,26. Schlosser et al were able to assess 74% of distal anastomoses with a 16-slice scanner. Single-source 64-slice scanners have improved our ability to assess distal anastomoses8,18,27. However, this site is particularly prone to false positive results which limits specificity. Although the assessment of distal anastomoses was not pre-specified in our study protocol, 92% were evaluable in the present study. Recent advances in technology and refinements in data acquisition and protocols have further increased the evaluable distal anastomoses12.
Our patients received a median effective radiation dose of 13.3 mSv. We must take into account that patients with a previous bypass operation receive more radiation since the scan target is broadened. A conventional coronary angiogram has a median radiation dose of 5.6 mSv. This has a risk of one in 3,600 of inducing a fatal cancer. CT angiography has a risk of one in 1,40028. However, patients with bypass receive more radiation during conventional angiography. Furthermore, a recent study suggested that radiation dose exposure is more favourable for MSCT than for conventional angiography in patients with bypass grafts in comparison to patients without bypass grafts29. While MSCT use is increasing for purely diagnostic reasons, physicians should be aware of the radiation dose and the factors that affect it, such as different scan protocols with less tube voltage and/or prospective ECG acquisition.
Limitations
The present study was designed as a proof-of-concept study to evaluate, non-invasively, the rate of early pre-discharge graft attrition in eligible and consenting consecutive patients in the modern era. Exclusion of patients with renal impairment and postoperative arrhythmia creates a bias towards patients with a favourable surgical outcome. Furthermore, the study is limited to single grafts, excluding jump grafts with multiple anastomoses, and patients without other non-coronary surgery. Given the documented accuracy of MSCT in demonstrating graft occlusion, comparison with conventional angiography was deemed unethical. Although the potential for MSCT to guide management in patients with persistent or recurrent symptoms post CABG is obvious, the number of patients in our study was too low to correlate graft occlusion with clinical endpoints. For the same reason, a multivariate analysis to assess predictors of graft occlusion was not performed. Finally, this was a single-centre study; therefore, results may not be directly extrapolated to other institutions.
Conclusions
Recently, Liu presented data regarding graft patency as evaluated with a 64-slice scanner. However, graft patency was evaluated at one month and four years18. To our knowledge, ours is the first study with dual-source 64-slice MSCT to report the in-hospital rate of coronary artery bypass graft occlusion. The results demonstrate that the in-hospital acute graft failure rate is 3.4% (6.8% of patients). The rate of in-hospital graft occlusion after coronary surgery is small but not negligible, and is mainly modulated by anatomical or procedural characteristics. The relatively low rate of acute graft attrition may be related to improvements in operative technique or to advances in background drug therapy. The in-hospital and subsequent course of the patients with graft occlusion was uneventful. While our study demonstrates the potential of MSCT to detect early graft occlusion, there is no evidence to support its systematic use in asymptomatic patients. Nevertheless, MSCT has potential as a robust technique to assess novel therapies to reduce the rate of graft attrition further and might be clinically useful to guide therapy in patients with persistent or early recurrence of symptoms after CABG.
| Impact on daily practice In-hospital, early graft occlusion after coronary surgery is a rare but clinically relevant issue and mainly modulated by anatomical or procedural characteristics. MSCT has potential as a robust technique to assess novel therapies to reduce further the rate of graft attrition. Although graft occlusion is usually clinically silent in certain scenarios, MSCT might be useful to guide therapy in patients with persistent or early recurrent symptoms after CABG. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

