
Abstract
Aims: The aim of this study was to assess prospectively the clinical benefits of fractional flow reserve (FFR) in guiding coronary artery bypass grafting (CABG).
Methods and results: GRAFFITI is a single-blinded, prospective, multicentre, randomised controlled trial of FFR-guided versus angiography-guided CABG. We enrolled patients undergoing coronary angiography, having a significantly diseased left anterior descending artery or left main stem and at least one more major coronary artery with intermediate stenosis, assessed by FFR. Surgical strategy was defined based on angiography, blinded to FFR values prior to randomisation. After randomisation, patients were operated on either following the angiography-based strategy (angiography-guided group) or according to FFR, i.e., with an FFR ≤0.80 as cut-off for grafting (FFR-guided group). The primary endpoint was graft patency at 12 months. Between March 2012 and December 2016, 172 patients were randomised either to the angiography-guided group (84 patients) or to the FFR-guided group (88 patients). The patients had a median of three [3; 4] lesions; diameter stenosis was 65% (50%; 80%), FFR was 0.72 (0.50; 0.82). Compared to the angiography-guided group, the FFR-guided group received fewer anastomoses (3 [3; 3] vs 2 [2; 3], respectively; p=0.004). One-year angiographic follow-up showed no difference in overall graft patency (126 [80%] vs 113 [81%], respectively; p=0.885). One-year clinical follow-up, available in 98% of patients, showed no difference in the composite of death, myocardial infarction, target vessel revascularisation and stroke.
Conclusions: FFR guidance of CABG has no impact on one-year graft patency, but it is associated with a simplified surgical procedure. ClinicalTrials.gov Identifier: NCT01810224
Introduction
Revascularisation of patients with stable coronary artery disease (CAD) can be justified either on account of significant documented myocardial ischaemia or because of persistent limiting symptoms despite the best available medical therapy1,2. In case of lacking or ambiguous non-invasive diagnostic functional tests, revascularisation of angiographically equivocal coronary stenoses should be limited to lesions responsible for reversible ischaemia based on invasive haemodynamic assessment by means of indices such as fractional flow reserve (FFR). This recommendation relies upon the fact that percutaneous coronary intervention (PCI) of lesions with FFR ≤0.80, irrespective of the angiographic severity, results in improved clinical outcome, overcoming the traditional way to assess stenosis severity and guide revascularisation based upon coronary angiography3. This concept has been extensively investigated and validated for percutaneous revascularisation; however, whether a systematic FFR assessment is also beneficial as compared to the traditional angiographic evaluation in guiding coronary artery bypass graft surgery (CABG) is still under evaluation.
The available data come from either retrospective or single-arm prospective registries. Botman et al4 showed that the functional severity of CAD determined preoperatively by FFR correlated significantly with graft patency at one year. Similarly, a significantly higher graft patency rate was observed with an FFR-guided versus angiography-guided strategy in a retrospective registry of patients treated with CABG. While no significant difference in major adverse clinical endpoints has been observed up to three years, a marked difference in death and myocardial infarction was only observed with an extended six-year follow-up5,6. Recently, the Fractional Flow Reserve Versus Angiography Randomization for Graft Optimization (FARGO) trial demonstrated (in 100 patients undergoing CABG in three Danish centres) no difference in both graft patency rate and clinical endpoints between angiography-guided and FFR-guided bypass grafting at six-month follow-up7. The GRAft patency after FFR-guided versus angiography-guIded CABG (GRAFFITI) trial assessed the impact of functional assessment of CAD prior to CABG on surgical strategies, graft patency and related clinical outcomes up to one year in a multicentre, multinational, prospective randomised trial.
Methods
HYPOTHESIS
The objective of the GRAFFITI trial was to assess the importance of the functional assessment of CAD prior to CABG. In particular, an FFR-guided strategy was compared to the angiography-guided strategy in the procedural planning and performance of surgical revascularisation. Based on previous data4, we hypothesised that the rate of graft patency in case of FFR-guided bypass surgery would be significantly higher at one-year follow-up than in case of angiography-guided bypass surgery. We also presumed that FFR guidance might be beneficial in terms of recovery and length of hospitalisation, mainly through a reduction in the need for more extensive surgical intervention (i.e., reduced number of grafts needed, reduced rate of on-pump surgical approach)5.
STUDY DESIGN
The study design has been published in detail previously8. The GRAFFITI trial is a single-blinded, open-label, prospective 1:1 randomised controlled multicentre, multinational trial, conducted in six European centres: the Cardiovascular Research Center Aalst – OLV Hospital (Aalst, Belgium), the University of Verona (Verona, Italy), the University Hospital of Brno (Brno, Czech Republic), the Hospital Santa Marta (Lisbon, Portugal), the Hungarian Institute of Cardiology (Budapest, Hungary), the Na Homolce Hospital (Prague, Czech Republic). The trial is registered at www.clinicaltrials.gov (NCT01810224).
Patients with stable CAD or non-ST-elevation acute coronary syndrome were enrolled after having signed the written informed consent form and if they met all the eligibility criteria, as follows: a significantly diseased left anterior descending (LAD) artery or left main stem (LM) by angiography or by FFR and at least one additional major coronary artery with an angiographically intermediate stenosis (30-90% diameter stenosis [DS] by visual estimate). Patients presenting with a myocardial infarction without ST-segment elevation could be included earlier than five days after the infarction if the peak creatine kinase level was less than 1,000 U per litre. Exclusion criteria were: acute ST-elevation myocardial infarction, moderate to severe valve disease with indication for valve surgery, severe left ventricular dysfunction (ejection fraction <35%), atrial fibrillation, and in cases where a Maze procedure was indicated. During the same procedure all intermediate stenoses were invasively assessed by FFR, but related values were kept concealed; the cardiologist involved in the procedure did not participate further in discussion on the revascularisation strategy. Included patients were then discussed within the Heart Team meeting where the surgeons involved were asked first to define their revascularisation strategy based on the coronary angiogram, including planned target vessels, type and number of grafts and anastomoses, need for heart-lung machine and possibility of a minimally invasive approach. Then patients were randomised to an angiography-guided or to an FFR-guided strategy. Patients randomised to the angiography-guided group were operated on following the initial angiography-based strategy without disclosing the FFR values at any stage. In cases where patients were randomised to the FFR-guided strategy, the measured FFR values were disclosed to the surgeons who were mandated to readjust the initially predefined surgical protocol according to the actual functional significance of each coronary stenosis, i.e., stenoses with an FFR ≤0.80 were to be grafted, while stenoses with an FFR >0.80 were to be left alone (Figure 1).
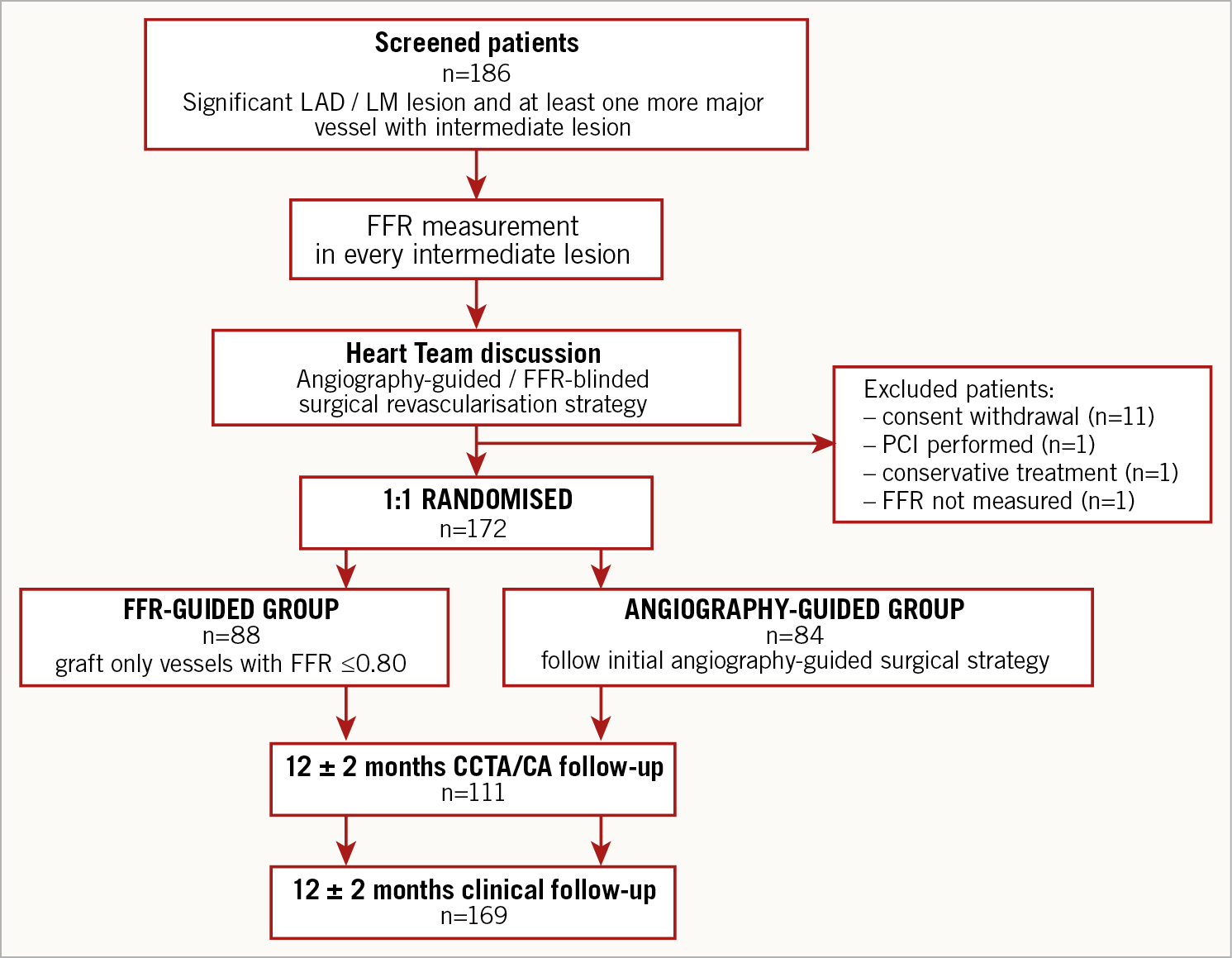
Figure 1. Flow chart of the GRAFFITI trial.
The study conformed to the principles of the Declaration of Helsinki, meaning that all patients signed a written informed consent stating that participation was voluntary and that participation could be withdrawn at any time without any negative consequences concerning their current or future medical treatment. Study approval was obtained from the local ethics committee of each participating centre.
ENDPOINTS
The primary endpoint of the trial was the rate of graft occlusion at 12 months. For evaluation, coronary computed tomography angiography (CCTA) was favoured9. All grafts were considered as patent or occluded. In case of clinically indicated coronary angiography (CA), graft patency was assessed by the angiographic examination according to good clinical practice. In the latter case, CCTA was not performed.
Secondary endpoints were (1) length of postoperative hospital stay, (2) changes in surgical strategy depending on FFR results (in the FFR-guided group only), and (3) rate of major adverse cardiac and cerebrovascular events (MACCE), i.e., a composite of death, myocardial infarction, stroke, or any revascularisation during the follow-up period.
STATISTICAL ANALYSIS
Based on the limited data available in the literature at the time of the study design4,5, an absolute difference of 10% was assumed in the rate of occluded grafts at one year between the two groups. Therefore, a sample size of 206 patients was calculated with 80% power, an alpha of 0.05 and presuming a 20% loss to follow-up.
The study analysis was on an intention-to-treat basis. All analyses were performed with GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA). Normal distribution of clinical and angiographic characteristics was tested with the D’Agostino-Pearson omnibus normality test. Here, continuous variables are expressed as mean±SD or as median (interquartile range), and categorical variables are reported as frequencies and percentages, as appropriate. The clinical and angiographic characteristics of the two groups were compared by unpaired Student’s t-tests, Mann-Whitney tests or the ANOVA test and categorical variables were compared with Fisher’s exact or chi-square tests, as appropriate. For the comparison of the adopted revascularisation strategies and surgical procedural characteristics, Fisher’s exact or chi-square or Student’s t or Mann-Whitney test was used, as appropriate. Differences in clinical endpoints, namely all-cause death, spontaneous myocardial infarction, target vessel revascularisation, stroke, and their composite (MACCE) were assessed by Kaplan-Meier curves. Results were adjusted by Cox regression multivariate analysis, when appropriate. A probability value of p<0.05 was considered as significant.
Results
PATIENT POPULATION
From March 2012 to December 2016, 186 patients were screened and 172 patients were randomised. Patients were excluded from randomisation for the following reasons: consent withdrawal (n=11), PCI performed (n=1), indication to conservative treatment (n=1), and FFR not measured (n=1). Due to slow recruitment, the steering committee decided to halt the enrolment of new patients, while completing the scheduled follow-up in 84 patients randomised to the angiography-guided group, and 88 patients randomised to the FFR-guided group.
Most of the patients presented with stable angina (89%). Clinical and angiographic characteristics are detailed in Supplementary Table 1. At baseline, there was no significant difference between the anginal status of the angio-guided and the FFR-guided groups (median of CCS classification 2 [1; 3] versus 2 [1; 2], respectively; p=0.278). The indication for invasive coronary angiography in patients with stable angina was not systematically documented. With the exception of previous myocardial infarction, there were no significant differences in clinical and angiographic characteristics between the two groups. Of note, normal FFR values were found in 27% of all measured coronary stenoses. The median values of both angiographic and haemodynamic metrics of the coronary stenoses were similar between the two groups.
ADOPTED REVASCULARISATION STRATEGY AND SURGICAL PROTOCOL
The final strategy adopted after randomisation is detailed in Supplementary Table 2. In the FFR-guided group, fewer bypass anastomoses were performed as compared with the angiography-guided group. In addition, a higher number of vessels with abnormal FFR (≤0.80) were bypassed and a higher number of vessels with preserved FFR (>0.80) were deferred in the FFR-guided versus the angiography-guided group. This translated into a higher rate of functionally appropriate targeted grafting (79% vs 68%; p=0.008 in the FFR-guided group) (Figure 2).
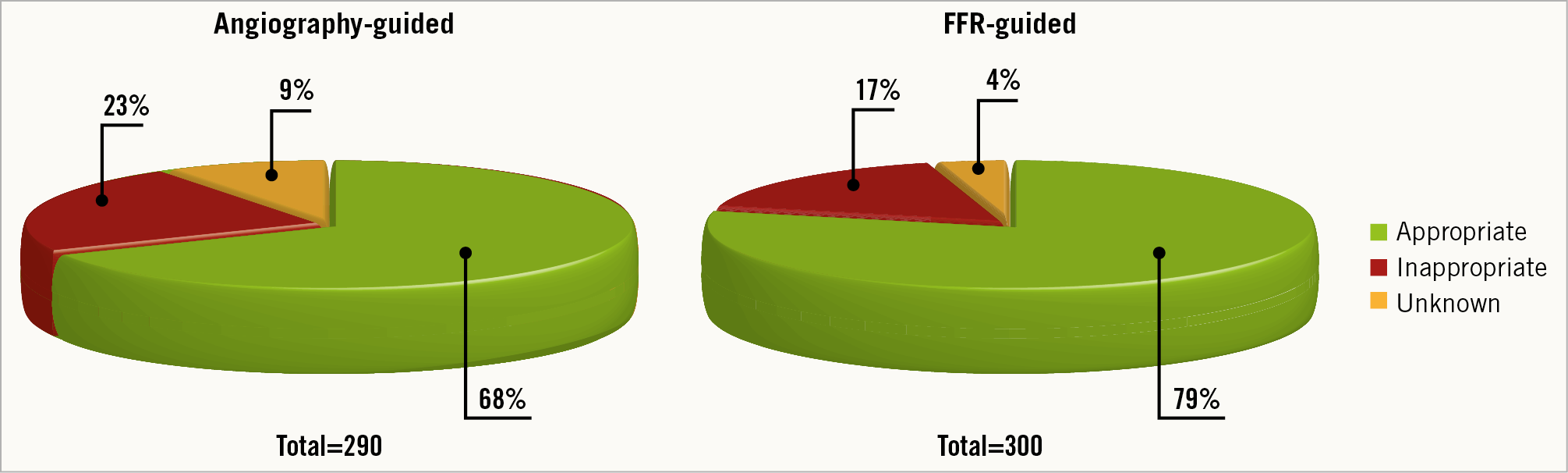
Figure 2. Adopted surgical strategies in relation to functional appropriateness.
The surgical protocol was also significantly different between the two groups (Supplementary Table 3). In the FFR-guided group, the median number of anastomoses per patient was significantly lower (2 [2; 3] vs 3 [3; 3], respectively; p=0.004), mostly due to a lower number of venous anastomoses per patient. This was also associated with a markedly higher rate of single-bypass revascularisations and a markedly lower rate of triple- or more bypass revascularisations. Off-pump (31% vs 14%, respectively; p=0.010) and minimally invasive surgery (10% vs 2%, respectively; p=0.036) were also more frequently performed in the FFR-guided group.
IN-HOSPITAL OUTCOME
Length of hospitalisation was similar in the angiography-guided and in the FFR-guided groups (11 [9; 14] vs 11 [9; 14] days, respectively; p=0.367). During hospitalisation there were no between-group differences in death (0 [0.0%] vs 1 [1.1%], respectively; p=0.327), in spontaneous myocardial infarction (2 [2.4%] vs 0 [0.0%], respectively; p=0.145), in target vessel revascularisation (2 [2.4%] vs 0 [0.0%], respectively; p=0.145), or in cerebrovascular events (0 [0.0%] vs 2 [2.3%], respectively; p=0.165).
FOLLOW-UP
One-year angiographic follow-up was performed in 56 (67%) patients in the angiography-guided group and 55 (63%) patients in the FFR-guided group. Follow-up was performed predominantly with CCTA (90.2%) and in the minority of patients with CA (9.8%). Angiography showed no difference either in the overall graft patency rate (126 [80%] vs 113 [81%], respectively; p=0.885) or in the graft patency of study vessels alone between the angiography-guided versus the FFR-guided groups (68 [73%] vs 54 [70%], respectively; p=0.733) at one year. Comparing native coronary arteries with patent versus those with occluded bypasses, we did not find any difference either in initial angiographic severity (DS 70% [60%; 80%] versus 70% [55%; 85%], respectively; p=0.599), or in functional severity (FFR 0.69 [0.50; 0.77] versus 0.65 [0.50; 0.79], respectively; p=0.764). Similarly, no correlation was found between graft patency and either DS (HR 0.992, 95% CI: 0.969 to 1.015; p=0.497) or FFR (HR 0.778, 95% CI: 0.051 to 9.278; p=0.778) in the native coronary artery disease. There was statistically no significant difference in the rate of arterial-to-venous grafts among the patent versus the occluded ones: 60/40% versus 47/53%, respectively; p=0.123. Protocol indicated only the measurement of vessel FFR; accordingly, we have no data on differences in graft patency between native vessels with focal versus diffuse disease.
Considering only the study vessels (i.e., excluding LAD territory that was significant per protocol), patency difference was found neither in the arterial grafts (50 [75%] vs 38 [73%], respectively; p=1.000) nor in the venous grafts (18 [69%] vs 16 [64%], respectively; p=0.771). Likewise, no difference was found in the patency of bypass anastomoses on stenotic vessels with FFR >0.80 (20 [77%] vs 11 [73%], respectively; p=1.000). The number of patients with at least one occluded graft was not different between the angiography-guided and the FFR-guided groups (17 [34%] vs 17 [37%], respectively, p=0.832).
One-year clinical follow-up was available in 83 (99%) patients in the angiography-guided group and 85 (97%) patients in the FFR-guided group. At the time of one-year follow-up, both groups showed marked improvement in anginal status without any difference between them: median of CCS classification 0 (0; 0) versus 0 (0; 0), respectively; p=0.620. During follow-up, no difference was found between the two groups in terms of MACCE, defined as the composite of overall death, myocardial infarction, target vessel revascularisation and stroke. Likewise, no statistical difference was found in the individual endpoints (Supplementary Table 4, Figure 3).
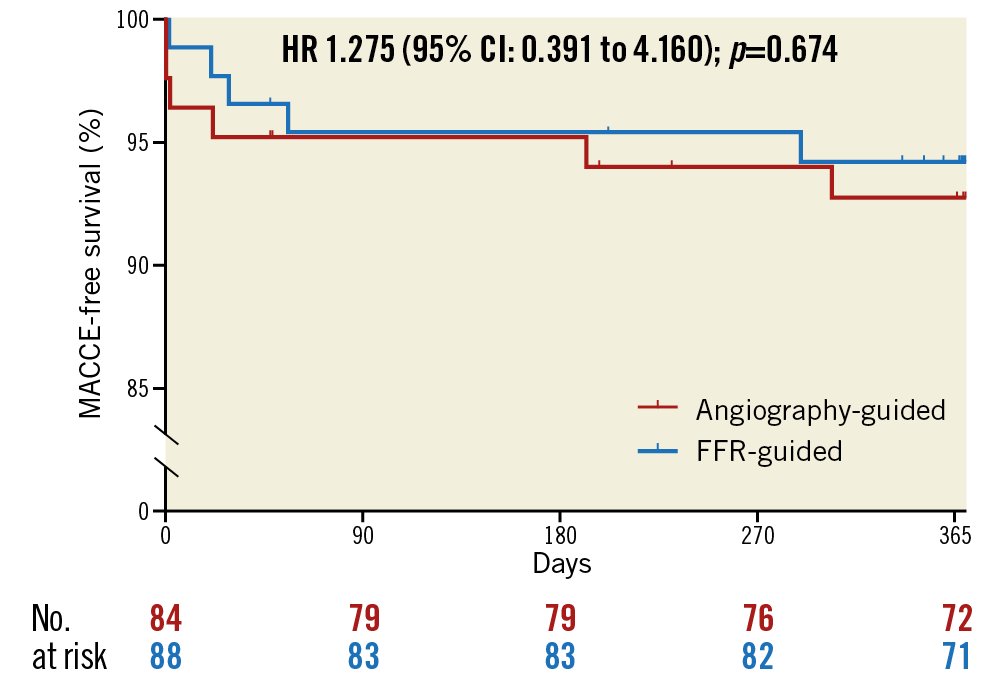
Figure 3. Incidence of major adverse cardiovascular and cerebrovascular events during one-year clinical follow-up.
Discussion
The clinical benefit of invasive functionally guided revascularisation has been proved for percutaneous coronary interventions. However, its impact still needs to be prospectively evaluated for the guidance of surgical coronary revascularisation10. GRAFFITI is the largest prospective trial investigating FFR guidance for patients undergoing CABG at a multicentre and multinational level in a randomised fashion8. Comparing an angiography-guided CABG group with an FFR-guided CABG group, the trial did not show a difference in the primary endpoint of graft patency rate at one year, as assessed by CCTA or CA. However, FFR guidance was associated with a significantly higher rate of functionally appropriate revascularisation, and a markedly simplified surgical strategy, namely fewer anastomoses. It also allowed a higher rate of off-pump and minimally invasive surgery. Importantly, the patients randomised to FFR-guided CABG, despite having fewer anastomoses, did not show any excess of cardiovascular and cerebrovascular hazard up to one year.
GRAFT PATENCY AFTER ONE-YEAR FOLLOW-UP
Despite the fact that the previous data used to calculate sample size suggested a 10% absolute difference in the rate of occluded grafts at one year4,5, the GRAFFITI trial failed to demonstrate such a difference and did not meet the primary endpoint. Yet, GRAFFITI confirmed and further extended up to one year the results of the recently published FARGO trial demonstrating no difference in graft patency at six months7. Our results can be explained mainly by the recruited study population, in whom three quarters of all coronary stenoses were functionally significant, therefore diluting the potential impact of a functionally guided strategy and eventually resulting in an underpowered study. Nevertheless, the GRAFFITI trial allows calculating the appropriate sample size of an adequately powered clinical trial to investigate graft patency in a randomised fashion, suggesting the need for 1,148 patients (574 patients per group), to be able to meet the primary endpoint.
ROLE OF FFR TO GUIDE SURGICAL REVASCULARISATION
Prognostic benefit of coronary revascularisation can be expected only in case of large, reversible myocardial ischaemia11. However, in complex coronary pathologies such as multivessel or LM disease which is mainly the case in candidates for surgical revascularisation, the diagnostic performance of non-invasive tests is limited: the poor spatial resolution barely allows distinguishing the impact of one vessel from the others12. On the other hand, the major advantage of invasive functional assessment, i.e., FFR, is its higher spatial resolution and its capability to interrogate the functional relevance of every single stenosis in isolation13. Considering the marked mismatch between the angiographic appearance and the true functional relevance of a coronary stenosis, namely the 50% DS conventional angiographic cut-off, this can result in a functional misinterpretation and potential “mistreatment” in at least one third of all cases, which is of the utmost clinical importance especially in multivessel disease patients14,15,16,17,18.
This was particularly evident in our study. Angiographically, there was no difference between the two groups, i.e., the number of lesions per patient was comparable. Still, after FFR assessment in the FFR-guided group, markedly fewer bypasses and anastomoses remained indicated, as compared to the pure angiography-guided patients. This reduction of indicated grafts primarily affected the number of venous grafts, resulting in a relative increase in the rate of arterial revascularisation. In addition, the simplified revascularisation strategies shown in the GRAFFITI trial associated with FFR implementation might help the surgeons to perform off-pump more often, as well as to perform minimally invasive surgeries. Even more importantly, FFR guidance is associated with higher functional accuracy of revascularisation strategies. Pure angiographic assessment in the angiography-guided group determined that almost a quarter of all bypasses were placed on vessels with non-ischaemia-inducing stenoses. Accordingly, taking the measured FFR value as reference (note, for the analysis FFR value was known for all the stenoses, regardless of the randomisation group), the rate of functionally inappropriate revascularisation strategies, including patients with grafted functionally non-signi-ficant vessels or patients with ischaemic territories left untreated, reached 62% in the angiography-guided group. This pure angiography guidance results either in patient overtreatment, i.e., more bypasses than necessary, or in undertreatment, i.e., fewer bypasses with ischaemic territories potentially left not revascularised.
Literature data suggest that similar benefit might be achiev-able by using non-hyperaemic indices in terms of appropriate functional guidance of revascularisation. This might result in broader acceptance of application in routine clinical practice, when planning surgical revascularisation.
FFR GUIDANCE AND CLINICAL OUTCOME
The overall simplified revascularisation strategies with markedly fewer anastomoses in the FFR-guided group as compared to the angiography-guided group (i.e., the functionally complete versus angiographically complete surgical revascularisation) did not show any signal of excess cardiovascular or cerebrovascular hazard up to one-year follow-up, confirming previous data from retrospective registries4,5.
Still, these findings allow a proper sample size calculation for a randomised trial investigating clinical outcome after angiography-guided versus FFR-guided bypass surgery. Almost 2,900 patients per group are needed for a properly sized non-inferiority design with MACCE as primary endpoint. Considering these figures, a large enough prospective randomised study would be difficult to perform.
Limitations
The trial has some limitations which must be acknowledged. 1) Enrolment was prematurely stopped due to slow recruitment; however, the results suggest that not even complete enrolment could confirm its findings better. 2) Angiographic follow-up is missing in a subgroup of patients. Nevertheless, as described above, the experienced clinical scenario resulted, by itself, in a certain undersizing to investigate graft patency. Therefore, a reliable assessment of difference in graft patency between these two groups could be achieved only in a sixfold larger group. 3) The trial was performed in centres with extensive experience with FFR-guided revascularisation. Accordingly, the practice of surgeons might also have been influenced by a certain experience with understanding anatomical-functional mismatch. This is reflected in the fact that “only” 61% of the angiographically significant but functionally non-significant vessels were bypassed in the angiography-guided group, diluting the potential graft occlusion risk in the reference group, although a much higher rate could be anticipated from conventional surgical approaches4. Remarkably, several revascularisation decisions in the FFR-guided group deviated from the functional indication, namely either no grafts to vessels with FFR <0.80 (29%) or grafts placed to vessels with FFR >0.80 (11%). For the first scenario, the causes were mainly technical, namely targets turned out to be non-graftable during surgery. The second scenario was caused by the reluctance in some cases to defer revascularisation of angiographically stenotic vessels, despite the fact that they were haemodynamically not significant5. While one year is appropriate for evaluating graft patency, the same might be too short to assess clinical outcome, especially taking into consideration recent literature data showing that outcome curves deviate around the third year of follow-up6.
Conclusions
Functional assessment prior to CABG had no impact on the graft patency rate. However, FFR guidance of CABG was associated with a significantly higher functional accuracy of the revascularisation and also a simplified surgical strategy with fewer anastomoses. A larger randomised clinical trial will be needed to assess superiority in the graft patency rate and non-inferiority in clinical outcome between FFR-guided and angiography-guided surgical revascularisations.
|
Impact on daily practice Routine FFR guidance of surgical revascularisation might allow marked simplification of the surgical strategy with fewer grafts and anastomoses. Although results do not show benefit in terms of graft patency, there was also no signal of hazard in functionally complete but angiographically incomplete revascularisation. |
Funding
This work was supported by a limited unrestricted grant (less than 15,000 euros) from Abbott Vascular/St. Jude Medical (St. Paul, MN, USA).
Conflict of interest statement
G.G. Toth has received consultancy fees from Abbott, Medtronic, Biotronik and Boston Scientific, not related to this study, and reports research grants and consulting fees from Abbott Vascular (St. Jude Medical), Medtronic, Boston Scientific and Biotronik. B. De Bruyne receives personal fees from Abbott, not related to this study, and reports research grants and consulting fees to the Cardiovascular Research Institute Aalst (Belgium) on his behalf from Abbott Vascular (St. Jude Medical). The other authors have no conflicts of interest to declare.

