
Abstract
Aims: Invasive coronary angiography (ICA) is more complex and challenging in patients with previous coronary artery bypass grafts (CABG). Computed tomography coronary angiography (CTCA) may provide useful information prior to ICA to improve these procedures. This study aimed to see if upfront CTCA prior to coronary angiography can reduce contrast load, procedural duration, and procedural complications compared to ICA alone.
Methods and results: This single-centre observational study included 835 patients with prior CABG undergoing invasive coronary angiography. One hundred and six patients underwent CTCA prior to ICA and were compared to 729 patients undergoing conventional coronary angiography alone (control group). No significant differences were seen between the two groups in patient demographics and procedural characteristics (number of bypass grafts), and interventional cardiologists’ experience. The CTCA group had lower contrast volumes (171.3 vs 287.4 ml, p<0.0001), radiation doses (effective dose 4.6 vs 10.5 mSv, p<0.0001) and procedure times (fluorosocopy time 9.5 vs 12.6 min, p<0.0001) at the time of ICA compared to patients who did not have prior CTCA. Combined radiation doses (ICA+CTCA) versus ICA alone were similar (p=0.867) with significant reductions in overall contrast used seen in the CTCA group (p=0.005). Complete diagnostic studies were performed in all patients with prior CTCA (106 patients, 100%) compared to 543 patients (74.64%, p=<0.0001) without previous CTCA. As a result, 34 patients (4.4%) went on to have CTCA post angiography due to missed grafts. Of these, four needed further invasive angiographic assessment and subsequent coronary intervention following the CTCA scan.
Conclusions: Prior CTCA improves graft detection at the time of coronary angiography and reduces the time necessary to localise graft ostium, the total procedure time, and volume of contrast media used.
Introduction
Each year, approximately 300,000 coronary artery bypass graft (CABG) surgeries are performed in the USA1 and 20,000 in the UK2. The purpose of CABG is to restore adequate blood supply to the heart; its success depends mainly on the patency of the bypass grafts3. Indeed, the natural history of bypass grafts is such that 10-15% of venous grafts are occluded per year, with 50% occluded after a period of 10 years4,5,6. This means that patients often need coronary evaluation by means of invasive coronary angiography (ICA), in order to explore recurrence of chest pain, heart failure, or for preoperative evaluation of cardiac valve replacement or high-risk non-cardiac surgery. While ICA remains the gold standard for coronary artery and graft evaluation, it is associated with a risk of complications such as kidney damage, myocardial infarction, stroke, bleeding, vascular complications and death7,8. In patients with a previous CABG, the risk of these complications is significantly increased9 since these patients are more fragile and have more comorbidities, such as older age and renal failure. In addition, ICA is more challenging in this setting, requiring multiple catheter manipulations to engage the grafts as the location of the bypass ostia is variable. Therefore, ICA in CABG patients lasts longer, leads to higher irradiation, more nephrotoxicity and is often performed femorally, which increases the risk of vascular complications. It is also associated with a greater incidence of complications than ICA in patients without CABG10,11. This emphasises the need for improvement in the techniques used to aid the search for bypass grafts with coronary angiography12,13,14,15.
Computed tomography coronary angiography (CTCA) has emerged as a useful clinical tool in CABG assessment, allowing detailed evaluation of venous and arterial grafts9 and the accurate detection of stenoses in bypass grafts9,16,17,18,19. This means that bypass grafts can be seen with minimal contrast (50 ml), time (<5 minutes) and radiation (<1 mSv), potentially optimising ICA in these patients. However despite a “may be appropriate” recommendation in US guidelines post CABG20, there are no data on how much a pre-ICA CTA may lower procedure time, fluoroscopy time or cumulative contrast load21. This study aimed to determine if upfront CTCA prior to coronary angiography can reduce contrast load, procedural duration, and procedural complications compared to ICA alone.
Methods
STUDY DESIGN AND PATIENT POPULATION
This was a single-centre cohort study conducted at Barts Heart Centre from September 2016 to February 2018. This was an observational study designed to assess the use of CTCA prior to ICA in this patient group, providing supportive data for further randomised study. All patients with previous single or multiple coronary artery bypasses who were listed for ICA were screened for prior CTCA and included in the study. For many of these patients, lack of graft notes was the primary indication; however, other indications included renal insufficiency, symptoms not typical of coronary ischaemia, and patient preference. The indication for the ICA was provided by the referring cardiologist; however, patients presenting with ST-elevation myocardial infarction (STEMI), or undergoing emergency invasive angiographic procedures (e.g., cardiogenic shock, cardiac arrest) were excluded from the study cohort. All CTCA patients who underwent physician-referred CTCA were identified and gave written informed consent to the use of the data for research purposes22. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and received approval by the relevant Institutional Review Board. Patients with CTCA performed prior to ICA were included in the CTCA group whereas patients without CTCA were included in the ICA alone group.
PROCEDURE/INTERVENTION
CTCA
All CTCAs were performed at Barts Heart Centre using a third-generation dual-source CT scanner (SOMATOM Force; Siemens Healthineers, Forchhiem, Germany) and ECG gating. The scan range included the inferior border of the heart to the subclavian arteries to visualise the origins of the grafts. Up to 40 mg of metoprolol was administered to achieve heart rate control, and scan acquisition type (e.g., high-pitch Turbo Flash, prospective and retrospective) was decided according to the individual patient as per protocol. The technical specifications of the scanner include a temporal resolution of up to 66 milliseconds (ms) with the use of two X-ray tubes to generate 384 slices. Siemens automatic kV selection programme (Care kV) was used to limit kV and therefore the radiation dose as per protocol. Contrast administration was weight based (4 ml/s with a total contrast volume of 51 ml for patients below 100 kg and 5 ml/s with a total contrast volume of 68 ml for patients above 100 kg).
IMAGE REVIEW
Images were analysed off-line using dedicated software (syngo Circulation; Siemens) by an experienced observer (>5 years’ experience in coronary CTA). The number of grafts present, their location and patency were recorded. Lesion characteristics were assessed using longitudinal and transverse sections and curved multiplanar reformats. Three-dimensional volume-rendered reconstructions of the grafts were created to aid angiographic assessment, optimised for easy visualisation of the graft ostia and trajectories. An example image is shown in Figure 1.
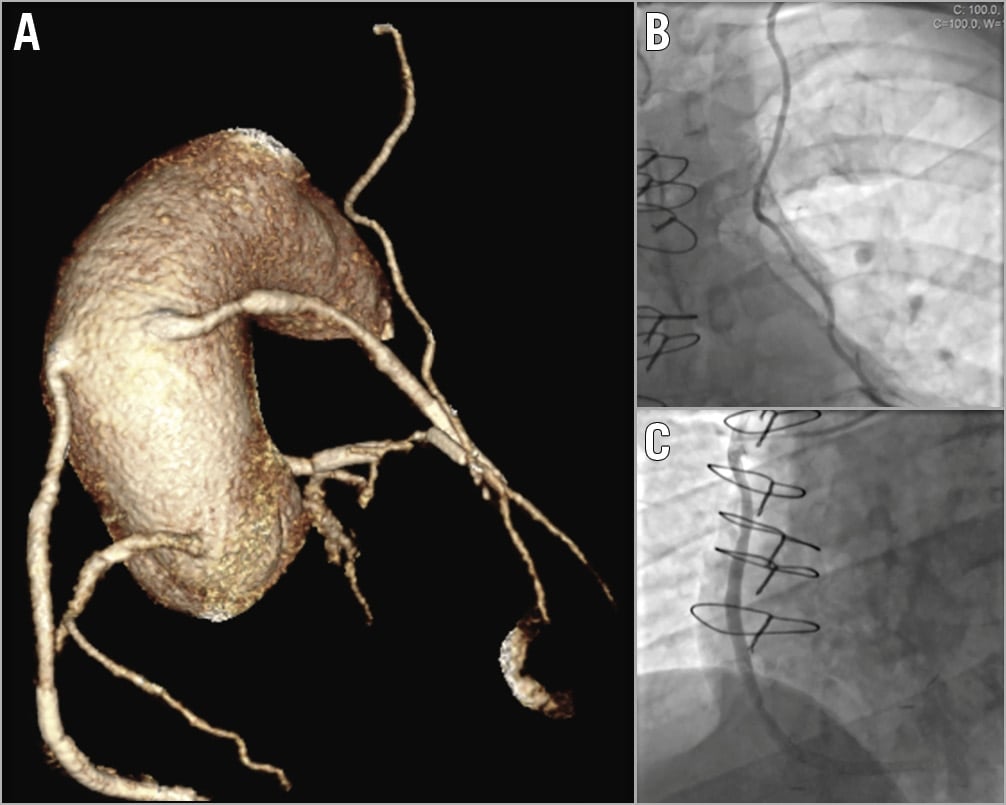
Figure 1. Example of CTCA reconstruction. A) A typical reconstruction from the CTCA of an example patient. It displays the origins of the native coronaries, a VG to the RCA (patent), VG-D1 (patent) and the LIMA (patent). Corresponding images of the LIMA (B) and VG-RCA (C) taken at ICA.
CARDIAC ANGIOGRAPHY AND PCI
The invasive procedures were a selection of diagnostic coronary angiography and percutaneous coronary intervention (PCI), all performed on Siemens Artis floor-mounted interventional angiography equipment. All machines were identical in terms of radiation exposure settings (fluoroscopy at 4 f/s, acquisition at 7.5 p/s, with dose per frame at 80 uGy/fr as standard); these factors were adjusted infrequently according to patient size. Contrast used was Visipaque (iodixanol strength 270 mg l/ml). The coronary angiographic procedures were performed by senior interventional cardiology trainees or interventional consultants, with their level of experience documented. Both the images and the CTCA report were available to the interventional cardiologist, providing the operator with information about the patency, number and location of the grafts.
It was not mandated that occluded bypass grafts on CTCA be selectively imaged on ICA; this was left to operator discretion. Grafts were considered occluded at ICA if stumps were found, or if the bypass graft was occluded on the CTCA or on prior ICA. Bypass grafts that were not found or seen during the ICA were considered unevaluated (i.e., incomplete studies), unless known to be occluded by prior ICA, and based on the interventional cardiologist’s opinion during the ICA. Further details of the interventional strategy and data collection can be found in Supplementary Appendix 1.
CT AND ANGIOGRAPHY RADIATION DOSE CONVERSION
In order to allow accurate comparison of radiation doses between CTCA and ICA, both dose area product (DAP) and dose length product (DLP) were converted to an approximate effective dose. For CTCA, a k-factor of 0.022 Sv·mGy−1cm−1 was used23 as per recent literature, whereas, for ICA, conversion rates can vary from 0.12 to 0.26 mSv/(Gy·cm2)24 dependent on the source; however, a unit of 0.22 mSv/(Gy·cm2) was used as per recent publications25.
ETHICS
Data were collected as part of a national cardiac audit and all patient-identifiable fields were removed prior to analysis. The local ethics committees advised that formal ethical approval was not required.
STATISTICAL ANALYSES
Baseline patient, procedural, and post-procedural characteristics were compared between the two groups. Categorical data are summarised using absolute values (percentage). Normally distributed, continuous data are presented as mean±SD or, where skewed, as median (25th to 75th centile). Normally distributed continuous variables were compared using Student’s t-tests, and the Mann-Whitney U test was used to compare non-normally distributed continuous variables. Categorical data were compared using the Pearson chi-squared test. A two-sided p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Between September 2016 and February 2018, a total of 835 patients with previous CABG underwent ICA and were included in the study cohort. Patients presenting with STEMI (82 patients) or undergoing emergency invasive angiographic procedures (cardiogenic shock [n=36], and cardiac arrest [n=33]) were excluded, resulting in the study cohort. Of the 835 included patients, 106 (12.7%) had CTCA prior to the ICA (CTCA group) and 729 did not (ICA alone group) (Figure 2).
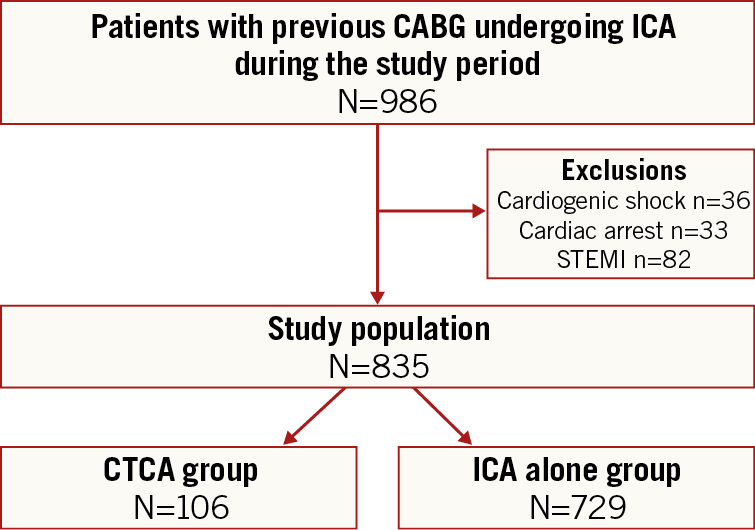
Figure 2. Study flow diagram. CTCA: computed tomography coronary angiography; CABG: coronary artery bypass grafting; ICA: invasive coronary angiography; STEMI: ST-elevation myocardial infarction
CTCA PARAMETERS
All 106 CTCA scans were well tolerated with no complications. The mean contrast dose was 60.5±6.4 ml, average radiation exposure calculated as effective dose was 6.0±1.3 mSv and median procedure time was 8.34 (IQR 7.1-10.2) minutes. All studies performed were deemed to be diagnostic with all grafts being visualised and quantified.
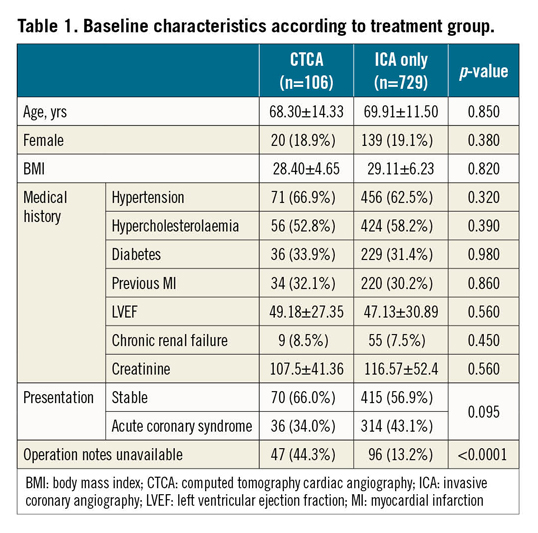
BASELINE CHARACTERISTICS (Table 1)
No significant differences were observed between the CTCA and the ICA alone groups in terms of age (68.30±14.33 vs 69.91±11.50 years, respectively; p=0.85), body mass index (BMI) (28.40±4.65 vs 29.11±6.23, respectively; p=0.82), and kidney function as assessed by the level of serum creatinine (107.5±41.36 vs 116.57±52.4 mol/L, respectively; p=0.58). Overall graft notes were unavailable in 143/835 patients (17.1%), with significant differences seen between the CTCA (44.3%) and ICA (13.2%) alone groups (p<0.0001).
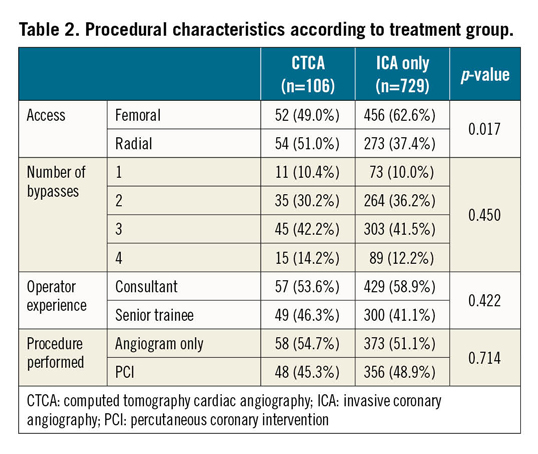
PROCEDURAL CHARACTERISTICS (Table 2)
The total number of bypass grafts to be located was not significantly different between the two groups (p=0.45), with a similar number of aortic anastomosis (p=0.23) and mammary grafts, allowing an unbiased comparison of the two groups. A similar number of known occluded grafts on prior ICA were present in the two study groups (6.7% vs 7.0%, p=0.63). Experience of the performing cardiologists was similar between the two groups with consultants performing the ICA in 53.6% versus 58.9% of the cases (p=0.42). A similar number of patients underwent PCI in the two groups, with comparable rates of procedural success (95.2% vs 96.6%, p=0.67).
ICA PROCEDURAL METRICS
The CTCA group had lower mean contrast volumes (171.3±50.6 ml vs 287.4±71.3 ml, p<0.0001), radiation doses (effective dose 4.6±1.5 mSv vs 10.5±3.3 mSv, p<0.0001) and procedure times (median fluoroscopy time 9.5 min [IQR 6.2-12.6] vs 12.6 min [IQR 9.1-15.6], p<0.0001) compared to patients who did not have prior CTCA. Despite a similar number of bypass grafts being found, the number of catheters used in the CTCA group was significantly lower than that in the ICA alone group (3.60±1.1 vs 2.32±1.0, respectively; p=0.002) (Figure 3).
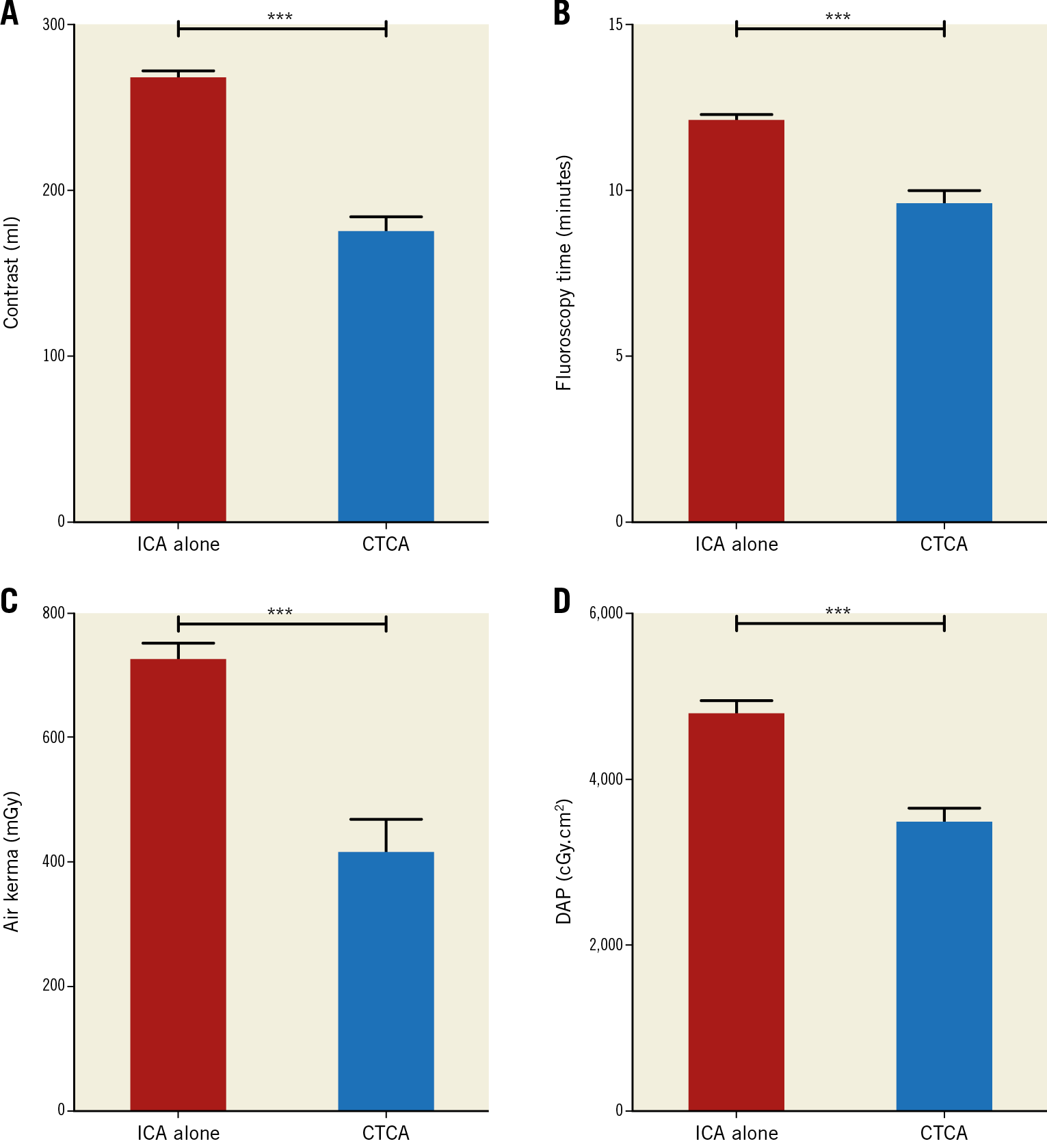
Figure 3. Angiographic parameters between the CTCA and ICA alone groups. A) The mean contrast amount comparing the CTCA and ICA alone groups, with the fluoroscopy time displayed in panel B. C) & D) The radiation metrics between the two groups showing air kerma and DAP, respectively. The mean contrast amount, fluoroscopy time and radiation doses were significantly lower in the CTCA group compared to the ICA alone group. Data shown as mean±SEM.
Complete diagnostic studies (i.e., all grafts seen with patency established) were performed in all patients with prior CTCA (106 patients, 100%) compared to only 74.6% (543 patients, p<0.0001) of patients without previous CTCA. In these incomplete studies the mean number of grafts not found was 1.80. This meant that in the ICA alone group 186 incomplete studies were performed with a total number of 335 grafts not found. As a result, 33 patients (4.4%) went on to have CTCA post angiography due to missed grafts. Of these, four needed further invasive angiographic assessment and subsequent coronary intervention.
COMBINED CTCA AND ICA METRICS
Patients who underwent CTCA prior to ICA received a combined total radiation dose of 10.7±2.8 mSv compared to 10.5±3.3 mSv in those who only underwent ICA alone (p=0.867). In terms of contrast, those undergoing both interventions still received a lower combined total amount (232.82±40.6 ml), compared to the ICA alone group (287.4±71.3 ml, p=0.015). Significant differences in access were seen between the two groups with a higher number of radial access routes in the CTCA group (51.3% vs 38.0%, p=0.017).
Discussion
This observational study demonstrates the contribution of pre-ICA CTCA in patients with previous CABG in improving the ICA procedure, both in terms of important interventional procedure parameters (procedure length, radiation, contrast amount and catheters used) and diagnostic information (patent or occluded grafts). This highly regarded technology provides a safe-to-deliver diagnostic tool for patients with previous CABG prior to (often difficult) coronary angiographic procedures. Although this is an observational study, with its inherent limitations, it provides a base for further study to demonstrate the efficacy of CTCA in post-CABG patients. Studies suggest that within three years of CABG one in five patients has required further coronary angiography 5,26, resulting in a large currently unmet need to improve this procedure. CTCA could provide the NHS with an option to improve the experience and outcomes of patients with previous CABG undergoing invasive coronary angiographic procedures.
The use of CTCA as a tool for preprocedural planning in invasive cardiology procedures has so far been largely limited to structural procedures such as transcatheter aortic valve implantation (TAVI). However, with massive improvements in image quality having been made in recent years, its use is growing in coronary angiography and PCI. The introduction of new third-generation dual-source CT scanners now means that bypass grafts can be visualised simply, as demonstrated in this study, allowing their potential use to plan interventional procedures21 27. Specifically in CABG patients, CTCA allows detailed evaluation of venous and arterial grafts and is highly accurate at detecting stenoses in bypass grafts with sensitivity, specificity, negative and positive predictive values of 97%, 97%, 93% and 99%, respectively9,16,17,18,19. Two recent systematic reviews, published in 2016, highlight the high sensitivity and specificity of CTCA for graft assessment. The first analysis of 31 studies (1,975 patients with 5,364 assessed grafts) demonstrated a sensitivity of 96.1% (95% CI: 94.3-97.4%) and specificity of 96.3% (95% CI: 95.1-97.3%) for bypass grafts, with higher sensitivity demonstrated for venous compared to arterial grafts (97.6% vs 89.2%, p=0.004)19. This was confirmed in the contemporary analysis by Barbero et al, assessing solely 64-slice CT scanning, where in 12 studies (1,586 grafts) the sensitivity and specificity for CABG occlusion were 0.99 (95% CI: 0.97-1.00) and 0.99 (95% CI: 0.99-1.00), respectively, with an area under the curve (AUC) of 0.9919.
While CTCA offers excellent accuracy for the detection of bypass graft stenosis or graft occlusion, this test is more limited for evaluating native vessels, where disease progression could be responsible for ischaemia, both in ungrafted native vessels, and in native vessels distal to the site of anastomosis21 28. The challenge when evaluating these vessels is that a substantial proportion has large amounts of calcified plaque, rendering them non-evaluable by CT due to blooming artefact. This limits the current ability of CTCA to replace ICA in this patient group; rather, it is a useful adjunct. If it were possible to visualise these native vessels sufficiently for potential assessment of ischaemia by CT using alternative functional assessments such as perfusion CT, then the value of CTCA as a stand-alone test to prevent unnecessary invasive evaluation could increase, i.e., it could replace ICA. However, to date, this technology has not undergone rigorous validation among patients with prior CABG, and current costs and logistics issues limit the adoption of this technology.
The results of this study are supported by the limited data in the literature which have assessed CT for guiding ICA in patients with previous CABG. One small observational study has assessed the use of pre-existing CT scans to create fusion images to guide cardiologists performing coronary angiography in patients with previous CABG15. The authors found that image fusion led to a reduction in procedural time, fluoroscopy time, amount of contrast administered, ionising radiation exposure, and procedural duration15. However, the study used pre-existing CT scans and it did not examine the clinical benefit of specific CTCA-guided ICA, which we have demonstrated for the first time. When we compared the combined radiation doses and contrast volumes of the CTCA and ICA in our patient cohort, radiation doses were comparable and, in the context of the CTCA group, spaced out, potentially reducing their effects. Perhaps more importantly, the skin dose in the CTCA cohort was significantly lower, a marker for much lower risk of radiation-induced skin injury. Prospective CTCA protocols could be optimised to reduce radiation and contrast doses further.
This study supports future randomised studies such as the BYPASS-CTCA study (NCT03736018) to assess whether routine CTCA in this patient group has the potential to be cost-effective and improve these difficult procedures. If the possibility that adequate pre-ICA planning thanks to CTCA could be responsible for higher rates of radial access alone, then it could mean that the approach may prove cost-effective29. Such study could potentially influence future clinical practice guidelines and provide the NHS with an option to improve the experience and outcomes of patients with previous CABG undergoing invasive coronary angiographic procedures.
Limitations
This study was an observational, prospective analysis of a large cohort of patients. The observational nature of this study may have led to confounding factors as is known with this study design. It is also a single-site retrospective study which has its own inherent limitations. The study also used convenience sampling where it could be argued that there may have been risks of selection bias; however, all possible attempts to avoid this have been made.
Conclusions
Prior CTCA has the potential to improve coronary angiographic procedures in the post-CABG patient, reducing the time necessary to localise grafts, total procedure time, and volume of contrast media. These findings have significant implications for how CTCA is integrated into the diagnostic algorithms of patients re-presenting with ischaemic heart disease (IHD) and previous surgical revascularisation.
|
Impact on daily practice Previous research has shown that invasive coronary angiography (ICA) is more complex and challenging in patients with previous coronary artery bypass grafts (CABG). Computed tomography coronary angiography (CTCA) has high sensitivity and specificity for graft visualisation and may provide a useful adjunct to ICA. This study showed that in patients where CTCA was used prior to ICA this resulted in reduced contrast volumes, radiation doses and procedure times compared to in patients who did not have prior CTCA. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

