
Abstract
Aims: Gender has been an important factor in outcomes after mitral valve surgery; however, its effect on percutaneous mitral valve repair is not well known. We aimed to report the effect of gender on outcomes in a large European prospective, multicentre, non-randomised post-approval study of percutaneous mitral valve repair.
Methods and results: Two hundred and five female and 362 male patients with significant mitral regurgitation underwent percutaneous repair at 14 European sites from October 2008 to April 2011. Women and men had similar baseline risk scores, but women had a higher rate of degenerative disease (32% vs. 18%). Women were more likely to have one clip implanted (72% vs. 54%), but had a similar length of stay in the intensive care unit (2.6±4.1 days) and overall length of stay (8.0±6.9 days) compared to men. They were also less likely to be discharged home: more women than men went to skilled nursing facilities (25% vs. 15%) and fewer women went home compared to men (71.9% vs. 83.9%). Thirty-day and 12-month safety results were similar between genders, as was 12-month efficacy (echocardiographic and clinical). Multivariate analysis showed no effect of gender on 12-month survival.
Conclusions: In a real-world, post-approval experience in Europe, female patients who underwent percutaneous mitral valve repair experienced safety and efficacy results similar to those of males. However, the discharge rates to skilled nursing facilities rather than home may indicate a need for better optimisation of the female patient’s physical and social comorbidities prior to intervention and during the hospitalisation period.
Abbreviations
ACCESS-EU: ACCESS-Europe. A Two-Phase Observational Study of the MitraClip System in Europe
CE: Conformité Européenne
EVEREST: Endovascular Valve Edge-to-Edge REpair STudy
MLHFQ: Minnesota Living with Heart Failure questionnaire
MR: mitral regurgitation
NYHA: New York Heart Association
PCWP: pulmonary capillary wedge pressure
6MWT: 6-minute walk test
TRAMI registry: Transcatheter Mitral Valve Interventions registry
Introduction
Severe mitral regurgitation (MR) is usually treated with reconstructive mitral valve surgery according to international guidelines1,2. Recent percutaneous therapies have also shown promise. MitraClip® therapy (Abbott Vascular, Santa Clara, CA, USA) is a percutaneous edge-to-edge therapy based on the technique of Alfieri3. Based on results from the Endovascular Valve Edge-to-Edge REpair STudy (EVEREST)4 randomised controlled trial comparing percutaneous therapy to mitral valve surgery, the MitraClip device has achieved guideline inclusion for both primary (class IIb recommendation in European and American guidelines) and secondary mitral valve disease (class IIb recommendation in European guidelines but not included in American guidelines). The MitraClip device received Conformité Européenne (CE) mark in March 2008.
Gender has been an important study variable in both mitral valve and cardiac surgery. Female gender has been found to be an independent risk factor for short-term mortality after coronary artery bypass graft surgery5,6 and for worse long-term outcomes in combined valve and bypass surgery7,8. In the case of mitral valve surgery, women have a different pathology compared to men, with decreased frequency of prolapse and more calcification of the posterior leaflet9. In contrast to both European1 and American2 guidelines, women are more likely to receive replacement as opposed to repair10 and may have worse long-term outcomes9.
The ACCESS-Europe A Two-Phase Observational Study of the MitraClip System in Europe (ACCESS-EU) study is a two-phase European prospective, multicentre, non-randomised post-approval study of MitraClip therapy. Enrolment and indication were based on physician clinical judgement. Initial one-year findings have been published11. The purpose of this study was to study gender-based differences in baseline characteristics, safety, and effectiveness in patients with significant mitral regurgitation treated with the MitraClip in the ACCESS-EU trial.
Methods
The ACCESS-EU Phase I post-approval study began enrolment in October 2008 and finished in April 2011. This study sought to obtain data from the use of the MitraClip system in the European Union with regard to safety and effectiveness in a real-world setting.
MITRACLIP PROCEDURE
The MitraClip system is a device used to treat mitral regurgitation. This percutaneous system utilises a polyester-covered cobalt-chromium clip to bring together the two mitral valve leaflets to minimise the effective orifice area and regurgitant volume. After initiation of appropriate anaesthesia, femoral venous access is obtained and transseptal access is undertaken under both echocardiographic and fluoroscopic guidance. The clip delivery system and catheter are brought through the transseptal access, over the mitral valve specifically at the area of the regurgitant jet, and down through the leaflets, where the clip is opened. The clip is then made to grasp the free edges of both leaflets, and echocardiographic analysis of MR reduction is performed. If MR reduction is adequate the clip is left in place, but if mitral regurgitation still persists the clip can be repositioned, removed, or implanted. Additional clips can be used based on the reduction of mitral regurgitation obtained, the underlying pathology and valve anatomy.
PATIENT SCREENING, ENROLMENT, TREATMENT, AND FOLLOW-UP
The decision to proceed with MitraClip therapy was made on a centre-by-centre basis, with specific attention given to a multidisciplinary Heart Team approach and approved labelling. Patients eligible for MitraClip therapy included those with moderate-to-severe (3+) or severe (4+) mitral regurgitation, as assessed by transthoracic and transoesophageal echocardiograms performed by the site at baseline.
All patients gave written consent and the study was approved by a local institutional review board. The study complies with the Declaration of Helsinki. Clinical assessment of symptoms with a six-minute walk test (6MWT) and Minnesota Living with Heart Failure questionnaire (MLHFQ) was performed at baseline, six and 12 months. Transthoracic echocardiograms were performed at baseline and 12 months after MitraClip treatment according to protocol.
Data collection in terms of demographic data, acute procedural results, and post-procedural follow-up data were collected by study personnel at investigational sites and entered into electronic case report forms. Echocardiographic studies and quantitative assessment of mitral regurgitation were performed at the study sites. All adverse events (AE) were site-reported events without prospective definition or adjudication by a clinical events committee. Data management was performed by MedPass International – ACCESS-EU Data Coordinating Center.
STATISTICAL ANALYSIS
Results were analysed for the entire ACCESS-EU cohort and additionally stratified into subgroups based on gender. Baseline and demographical qualitative variables were expressed as percentages, and quantitative variables were expressed as mean (SD) or median (25th-75th interquartile range). Serial paired data are shown for surviving patients only. Mitral regurgitation severity and New York Heart Association (NYHA) functional class were compared between baseline and 12 months using Bowker’s test. The null hypothesis (Ho: Pij=Pji) states that the marginal probabilities for each outcome (i.e., improvement and worsening) are the same. Rejection of the null hypothesis is evidence of lack of symmetry (Ho: Pij≠Pji), and a resulting statistically significant p-value indicates patients improved category following baseline. Changes in 6MWT and MLHFQ between baseline and 12 months were analysed using paired t-tests. Survival rates to 12 months were presented as Kaplan-Meier curves. Baseline variables which were statistically different between genders would be analysed further using Cox univariate regression to determine whether they were predictors of 12-month survival. Variables with p<0.25 were entered into a Cox multivariate regression analysis. Differences were considered statistically significant at the 0.05 level. The data were analysed with SAS statistical software, version 9.1.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Between October 2008 and April 2011, a total of 567 patients with 3+ or 4+ mitral regurgitation were enrolled at 14 European sites. The patients included 205 (36.2%) women and 362 (64.8%) men. Baseline characteristics by gender are shown in Table 1, including demographics and comorbidities. Women were more likely to be older, with a mean age of 76±8 years, compared to men with a mean age of 72±10 years. The female population was likely to be shorter with a smaller body surface area (1.7±0.2 m2) and less weight; however, body mass index was similar between the two genders. Coronary artery disease, myocardial infarction, prior cardiovascular surgery, cerebrovascular disease, chronic obstructive pulmonary disease, and moderate-severe renal failure were all less likely in women than in men. Women receiving the MitraClip were more likely to have degenerative mitral regurgitation than functional mitral regurgitation compared to men receiving the MitraClip procedure, but there was no difference in NYHA class, cardiogenic shock, or mitral regurgitation severity. However, the overall logistic EuroSCORE was similar between the two groups, which may be due to the other factors being a higher contributor to the eventual score.
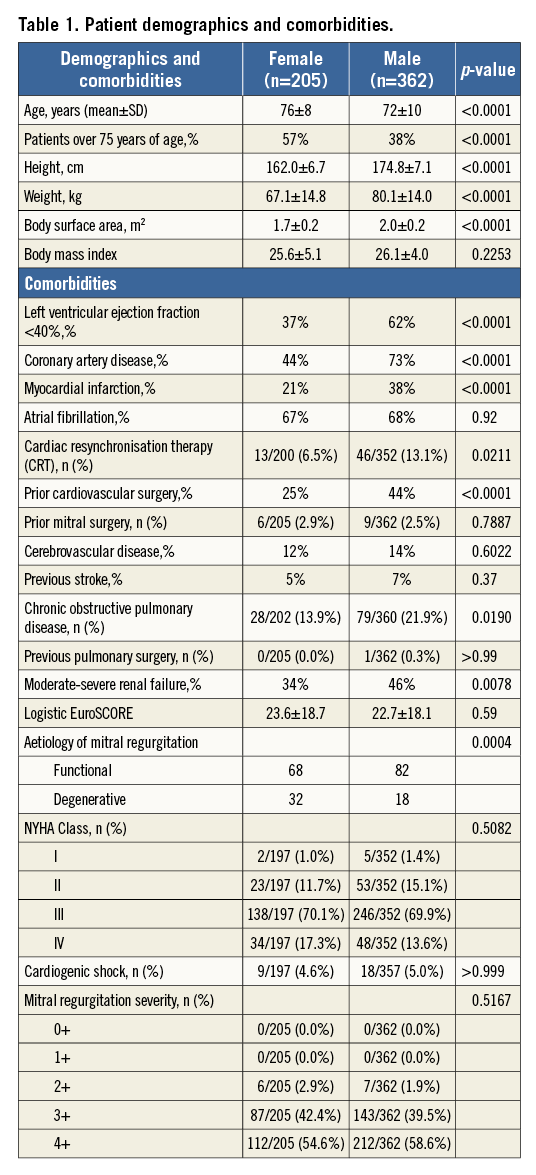
Procedural and hospitalisation information are shown in Table 2. Procedures were performed with a high implant success rate in both men and women (over 99%). Female patients tended to have one clip implanted as opposed to two (72% vs. 27%) compared to the male patients, who had a more equal distribution of one and two clips implanted (54% and 42%, respectively). There was also a trend towards an increase in cardiac output and decrease in pulmonary capillary wedge pressure in both genders. Although procedure time and contrast use was similar, fluoroscopy duration was less in women compared to men (27±19 min vs. 31±20 min, respectively).
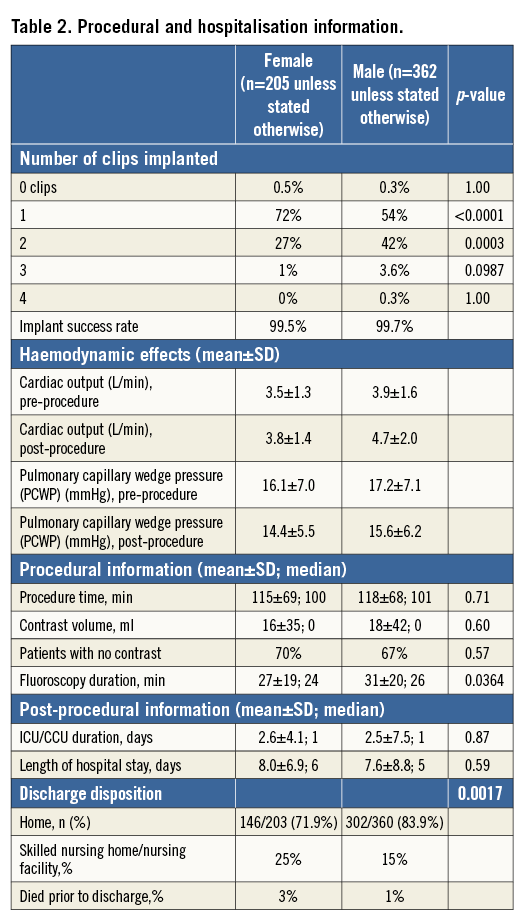
After the procedure, the duration of stay in the intensive care unit and the overall length of stay in hospital was similar between men and women. However, discharge disposition was remarkably different between genders. Men were more likely to go home compared to women (84% vs. 72%); women were more likely to go to a skilled nursing facility compared to men (25% vs. 15%).
At thirty days, centres reported safety events to the ACCESS-EU registry. Events can be found in Table 3. There were more deaths in the female group (5.4% vs. 2.2%) at 30 days, which was not significant, with approximately 56% being prior to discharge. There was a trend to more bleeding complications in the female group compared to the male group. Overall, safety events at 30 days did not vary significantly according to gender.

At 12 months, clinical information was collected, including mitral regurgitation severity, 6MWT, NYHA functional class, and MLHFQ Quality of Life score. At 12 months, there was a decrease in mitral regurgitation in both men and women, with 80% of men and 76% of women having a mitral regurgitation grade ≤2+ (value between groups p<0.40). The 6MWT was also improved in both women and men by over 50 metres in each group. Both groups had a significant decrease in NYHA class, with men having a larger decrease than women (p=0.0095). However, when this was analysed by MLHFQ Quality of Life questionnaire, the improvement was statistically similar in both groups, with a trend towards more improvement in women (Figure 1).
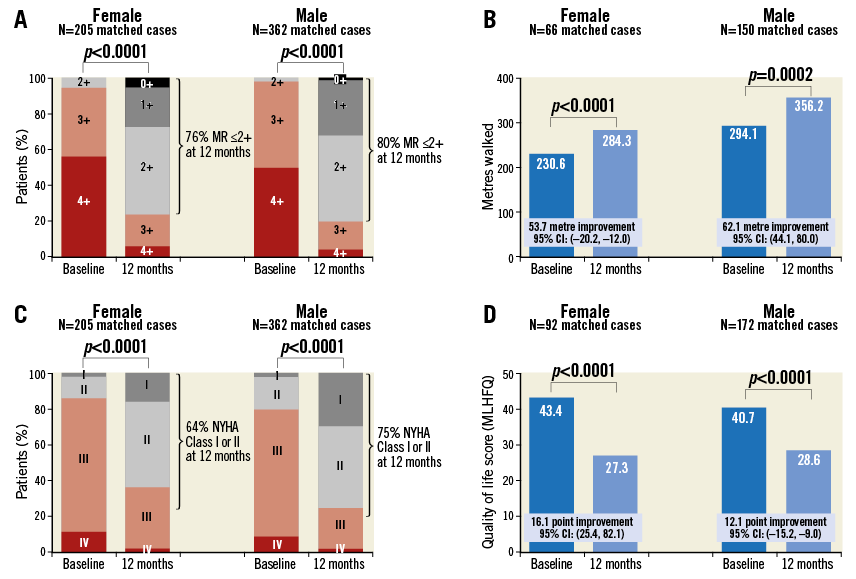
Figure 1. 12-month changes in men and women after MitraClip therapy. The figure is a composite of changes in mitral regurgitation grade (A), six-minute walk distance (B), NYHA functional class (C), and MLHFQ Quality of Life score (D) at baseline and 12 months after MitraClip procedure divided by gender. There was significant improvement in mitral regurgitation grade, six-minute walk distance, NYHA functional class, and MLHFQ Quality of Life score in both men and women at 12 months.
A small group of patients from the 205 women and 362 men required a second intervention. Of these, seven women (3.4%) and 12 men (3.3%) received a second MitraClip procedure (p>0.99). Mitral valve surgery was performed in 15 women (7.3%) and 21 men (5.8%), which was also not statistically significant (p=0.48). Of note, the rate of reported single leaflet device attachment was not different between groups (eight women and 19 men, p=0.54).
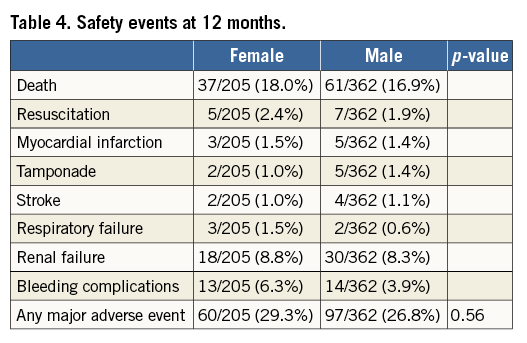
At twelve months, sites reported safety events in 205 female and 362 male patients. Safety events can be found in Table 4. At 12 months, there was much more similarity in adverse events between genders, including death (18.0% vs. 16.9% for women and men, respectively). Overall, safety events at 12 months did not differ between men and women.
At one year post-procedure, overall survival was similar for men and women (82.2% and 81.0%, respectively, p=0.60). The Kaplan-Meier curve is seen in Figure 2. Although the curves appeared to separate slightly at six months, this was not seen at one year. Univariate Cox regression analysis showed that only three variables were significant with a p-value to be included in the analysis: age, renal disease, and chronic obstructive pulmonary disease. The aetiology of mitral regurgitation was not significant.
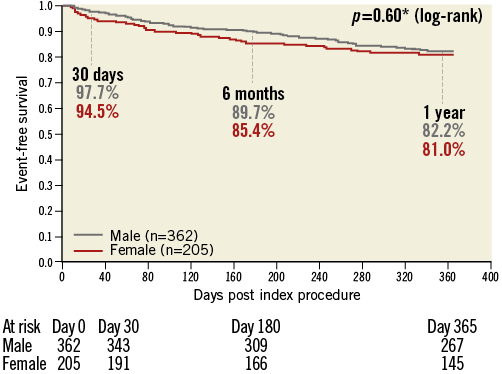
Figure 2. Kaplan-Meier event-free survival at 12 months by gender. At one year, event-free survival was 82.2% for men and 81.0% for women. This was statistically equivalent for both genders.
However, after adjusting for these three variables, there was no statistically significant gender effect on 12-month mortality (hazard ratio for gender 1.117, confidence interval 0.0728-1.713, p=0.6126).
Discussion
Based on scientific evidence from randomised controlled trials and also inclusion in European guidelines, the application and acceptance of MitraClip as an appropriate therapeutic option for select patients has increased. The ACCESS-EU registry compiled these data from 14 large European centres, allowing the capability to analyse specific subsets for important trends. One such trend is that of gender and the impact of the device. The role of gender in outcomes after mitral valve surgery compared to outcomes for percutaneous repair of mitral regurgitation has not been evaluated. The effect of gender on patients treated with the MitraClip yields interesting results: there is significant similarity in the long-term efficacy and safety data between the two, despite women who received the MitraClip having a smaller body surface area and a higher proportion of degenerative mitral valve disease than their male counterparts. There are similar decreases in echocardiographic mitral regurgitation severity and improvements in functional class/quality of life between both groups. However, women are more frequently discharged from the hospital to skilled nursing facilities rather than home.
Degenerative mitral valve disease has a different presentation in women versus men. Among patients presenting with mitral valve prolapse, there is decreased frequency of posterior prolapse, flail, severe regurgitation and increased leaflet thickening12.
When women and men are matched based on severity of mitral regurgitation, women are found to have smaller ventricular and atrial dimensions12,13. This is expected given the average smaller body habitus of female patients. European guidelines use cut-off values of left ventricular end-systolic diameter of 55 mm, 45 mm, and 40 mm1. Therefore, it is more likely that women may reach these dimensions as a result of more advanced disease or later presentation.
In the context of conventional mitral valve surgery, women are found to receive different treatment than men. In a study of Medicare beneficiaries aged 65 years and older, female patients were less likely to receive repair than men at an odds ratio of 0.63 (95% confidence interval 0.61-0.66)14. The increased lifespan of women may explain the same study’s finding that elderly patients less often underwent mitral valve repair. Female patients may also have a higher death rate after a mitral valve operation, as seen in the Medicare beneficiary data10, suggested by a worse in-hospital mortality rate (7.7% for women vs. 6.1% for men). However, the long-term survival rate was similar after risk adjustment. When this group of Medicare patients was compared to a general population of the same age and gender, mitral valve repair restored life expectancy in men but not in women. This trend for a decreased number of repair operations and higher short-term mortality rates in female patients may be due to later or delayed presentation, diagnosis of “guideline-indicated” operable mitral regurgitation, increased difficulty with repair in patients with smaller anatomies, and the presence of comorbidities with lack of caregiver support.
Women have been included in all the clinical trials for MitraClip. The Endovascular Valve Edge-to-Edge REpair STudy (EVEREST) I trial15 was a prospective multicentre single-arm study enrolling 41/107 (38%) women. The EVEREST II4 randomised controlled trial compared treatment with the MitraClip device to surgical treatment, enrolling 69/184 (37.5%) women in the MitraClip arm. Gender-specific results of the EVEREST I and EVEREST II trials are yet to be published; however, there were no differences in the primary endpoint at 12 months based on gender in the EVEREST II trial4. Of note, preliminary data from the German Transcatheter Mitral Valve Interventions (TRAMI) registry of 476 patients (200 women, 41% of the total) found that women were on average four years older than the men in the cohort and were less likely to have decreased LV function but still had an overall higher logistic EuroSCORE I score (23% for women vs. 21% for men). Women required fewer clips (1.3 vs. 1.5) in the procedure, which may also have contributed to the decreased procedural duration. This may be due to valve anatomy or difference in annulus size. However, there was no difference in safety or short-term 30-day efficacy between genders in this registry.
ACCESS-EU confirms the prior findings from the clinical experience, but in addition recognises that women often present with a different mitral valve pathology for degenerative disease and therefore have a different presentation of disease from men. Women are less likely to have certain comorbidities, e.g., coronary artery disease, myocardial infarction, cardiac resynchronisation therapy, prior cardiovascular surgery, cerebrovascular disease, chronic obstructive pulmonary disease, and moderate-severe renal failure. From a mitral valve standpoint, women in this cohort were found to have higher numbers of degenerative valve disease compared to men.
Results from this trial are in line with the previous MitraClip data showing equal safety and efficacy in men and women. However, the finding of discharge disposition is an important new addition to our clinical knowledge. Of note, there was also a trend towards increased 30-day mortality for women compared to men in this study (which was not as strongly present at the one-year mark). This occurred despite their having lower rates of recognised comorbidities. There are multiple possible causes. These include older age, higher rates of degenerative disease, unrecognised or non-optimally treated comorbidities (e.g., pulmonary hypertension), procedural issues (vascular issues from large-bore venous access, smaller body surface area leading to possibly higher post-clip gradients which, of note, were not collected in this registry), biochemical changes after the procedure (a registry study of almost 25,000 non-high-risk patients showed higher mortality after traditional mitral valve surgery in perimenopausal women compared to post-menopausal women16), different intensive care management (perhaps a less aggressive push to mobilisation), symptom minimisation (less likely to describe pain or heart failure symptoms during hospital stay), delayed psychological recovery, social setting issues (absence of a qualified primary caregiver at home). Women may have more depressive symptomatology17, functional limitations, lower life satisfaction, and social support compared to men after cardiac surgery18.
Whether any of these explains the finding of differential disposition after MitraClip procedure is speculative at this time. This does not mean that women should be sent home more often after the procedure; however, this may reflect a need for more thorough patient evaluation prior to the procedure. Perhaps more efforts should be made to ensure that all patients, especially women, are fully optimised prior to a MitraClip procedure from both a physical and a social care standpoint. There may also be a role for gender-specific or body surface area cut-off points in the guidelines. However, this study is a registry study, and other variables such as prior medical therapy, transmitral gradient, and pulmonary hypertension may play a very significant role in explaining these findings.
The rate of second intervention (clip or surgery) was also equivalent between men and women. The rate of second clip procedure at one year (slightly over 3%) is similar to the 2.8% in the EVEREST II trial19. Surgery for mitral regurgitation after MitraClip implantation was 20.8% (37/178) at one year in the same trial; this was much higher than the rate of 7.8% for women in this cohort. As mentioned in the original ACCESS-EU paper11, this rate may underestimate the proportion of patients with recurrent/residual mitral regurgitation due to the risk of surgery.
The findings from this study should be viewed in the context of the design of the study as an international multicentre all-comers European registry. There was no core lab adjudication of echocardiographic parameters, with an absence of morphologic valve evaluation or ventricular dimension changes reported. Data and events are self-reported, with a not insignificant loss to follow-up at the one-year mark. As this was a post-market study, there were no predefined enrolment criteria which may reflect site-to-site differences in indication or anatomical eligibility. Patients were included in the study based on receiving MitraClip therapy, not on specific indication or anatomic criteria. There was also no pre-specified medical therapy strategy, which may have affected outcomes. There may be other confounding variables present, such as height, weight, or body surface area. Given the sample size, further breakdown of the groups into subgroups for interaction and multiple testing may not be indicated. The finding of increased discharge to rehabilitative facility or skilled nursing home is at best exploratory.
Conclusions
Women with severe mitral regurgitation who are candidates for the MitraClip procedure should expect a similar safety (with respect to adverse events) and efficacy (with respect to regurgitation severity as well as clinical measures such as NYHA functional class, improvements in physical capacities, and quality of life). However, there are data to show that women may be leaving the hospital for non-home facilities, reflecting the need for better patient selection and optimisation, especially with regard to physical and social characteristics. In both daily practice and clinical studies going forward, the role of gender should be addressed further to help improve outcomes.
| Impact on daily practice Women and men achieve equivalent safety and efficacy results with the MitraClip procedure in a real-world setting. Women are more likely to have one clip implanted, and have a similar length of stay. However, increased discharge rates to skilled nursing facilities rather than home for women as compared to men may point to a need for better optimisation prior to procedure. |
Funding
The ACCESS-EU registry has been sponsored by Abbott Vascular.
Conflict of interest statement
S. Gafoor and H. Sievert’s institution has received study honoraria, travel expenses, and consulting fees from Abbott Vascular. F. Maisano has received consulting fees from Abbott Vascular. S. Baldus has received research grants and lecture fees from Abbott Vascular. U. Schaefer has received consulting fees from Abbott Vascular. J. Hausleiter has received speaker honoraria from Abbott Vascular. C. Butter has received research grants from Abbott Vascular. W. Schillinger has received lecture fees and reimbursement for travel expenses from Abbott Vascular. O. Franzen has received research grants, proctoring honoraria, and lecture fees from Abbott Vascular. The other authors have no conflicts of interest to declare.

