
Mitral regurgitation (MR) is the most common valvular heart disease in the Western world. Nearly one in 10 people aged ≥75 years is estimated to have moderate or severe MR1. However, ≈50% of patients with severe MR with an indication for surgery are turned down due to increased perioperative risk owing to advanced age, frailty, left ventricular dysfunction, and comorbidities2. This unmet need has led to the development of several percutaneous mitral valve repair and replacement technologies over the last decade3. Among the different devices available, the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) repair system has become the most widely used. Since the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) trial4,5 demonstrated its safety and feasibility, the use of the MitraClip has also been expanded to complex anatomies beyond the so-called anatomical “EVEREST criteria”.
Although the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines recommend consideration for transcatheter mitral valve repair (TMVr) with the MitraClip system (class IIb recommendation) only in patients with severe symptomatic degenerative MR (DMR) with prohibitive surgical risk6, the latest iteration of the European Society of Cardiology (ESC) guidelines recommends TMVr (class IIb) in high-risk patients with either severe DMR or functional MR (FMR)7. Consistent with the guideline recommendations, the practice patterns in TMVr adoption have differed considerably between Europe and the United States of America (USA). Data from the Transcatheter Valve Therapy (TVT) registry show that ≈85% of patients undergoing MitraClip implantation in the USA have DMR8. Contrarily, large European registries such as the ACCESS-EU, Transcatheter Valve Treatment Sentinel Pilot Registry (TCVT), and TRAMI (German transcatheter mitral valve interventions) have reported ≈75% of TMVr cohorts to have predominantly FMR9-12. Despite differences in the aetiology and baseline features of the study cohorts, these registries have shown excellent short- and long-term safety and efficacy outcomes with use of the MitraClip in real-world clinical practice (Table 1).
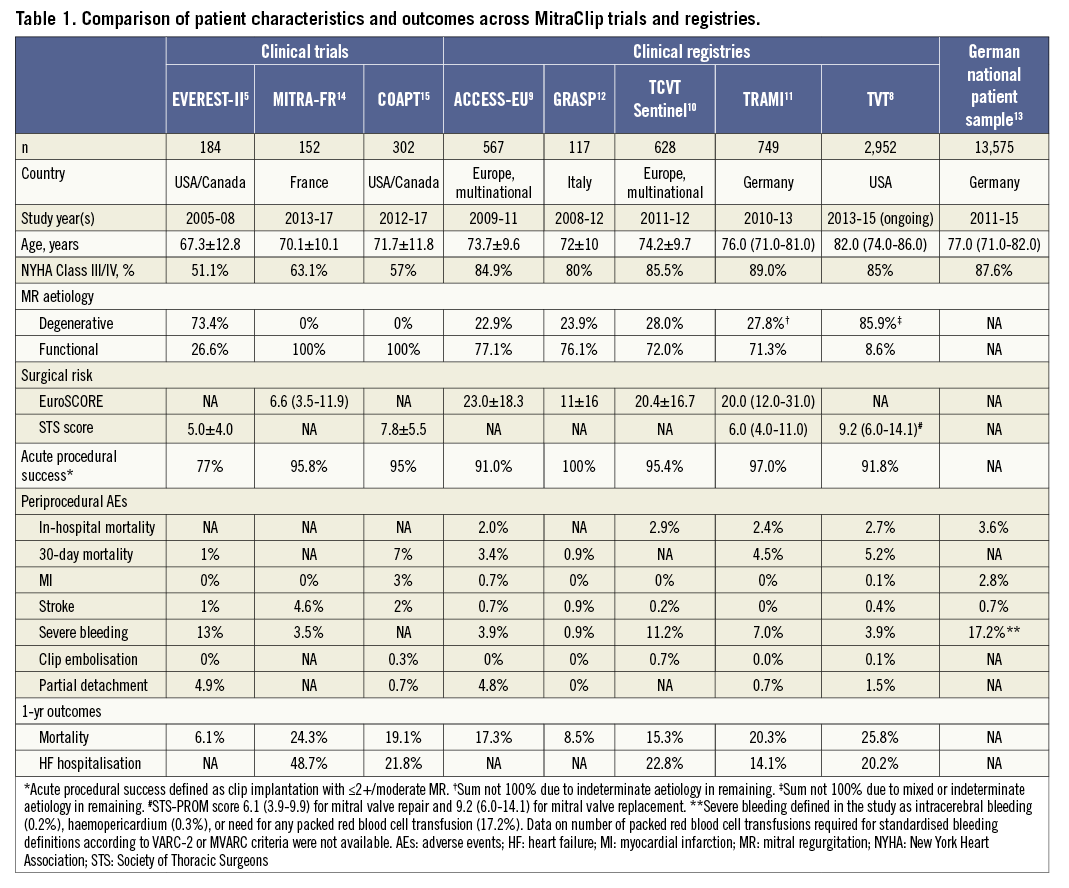
In this issue of EuroIntervention, von Bardeleben et al13 report the trends in utilisation, patient characteristics, and in-hospital safety outcomes of the MitraClip device using administrative data from the German Nationwide Inpatient Sample.
The main findings of the study were as follows:
1) Overall, 13,575 patients underwent MitraClip implantation in Germany from 2011 to 2015, with a fivefold temporal increase in the annual number of device implantations from 815 in 2011 to 4,432 in 2015.
2) The authors report a temporal increase in the mean age of the patients, comorbidity burden, and the proportion of those with NYHA III/IV heart failure (HF), reflecting a sicker study population over time. Despite the worsening risk profile, there was no temporal change in in-hospital mortality or major adverse cardiovascular event rates after multivariable risk adjustment.
3) Patients with advanced HF, cardiogenic shock, and those with procedural complications such as pericardial effusion, stroke and blood transfusion, had higher in-hospital mortality.
Even if the study represents the largest available cohort of patients treated with the MitraClip, the study is limited by lack of critical data such as aetiology of MR (functional vs. degenerative), validated risk scores, i.e., STS/logistic EuroSCORE, and echocardiographic parameters. Also, there are no efficacy data available on acute procedural success or degree of MR reduction, procedural complications such as clip embolisation or single leaflet attachment, or need for additional percutaneous/surgical procedures. Lastly, important long-term efficacy data such as degree of MR at follow-up, functional improvement, follow-up mortality, HF hospitalisation, etc., could not be assessed. However, the authors demonstrate that procedural complications such as cardiac tamponade, bleeding and stroke have a negative impact among this frail population, thus affecting short-term mortality negatively. Nevertheless, the authors should be congratulated for their excellent study which reaffirms that MitraClip implantation is being performed commercially with acceptable safety outcomes.
Recently, the Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation (MITRA-FR) trial reported that, in patients with severe FMR, MitraClip implantation was not associated with a reduction in all-cause death or HF hospitalisation at one year14. However, the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial showed that MitraClip therapy significantly reduced both all-cause mortality and HF hospitalisation at two-year follow-up15. The marked differences observed in the two trials are attributable to patient selection, with a degree of MR that was more disproportionate to the grade of LV dysfunction and dilatation in the COAPT study16. Cardiologists are trying to get a better understanding of where the “sweet point” for MR correction exists that will potentially allow TMVr technologies to improve the outcome of patients suffering from HF and concomitant MR. In this perspective, the study by von Bardeleben et al has to be considered a large “real-world” experience on the use of the MitraClip without restrictions on patient selection, confirming that use of this device over the inclusion criteria proposed by the trials is safe and feasible. Moreover, even when challenging patients are treated, the in-hospital mortality remains acceptable. Even though no clear data are available in the paper, increasing operator experience and improved management of patients with MR undergoing TMVr probably contributed to the favourable in-hospital outcomes observed in the study. In the future, “post-COAPT” registries are needed to understand whether better patient selection can effectively have an impact on the in-hospital and long-term outcomes of patients with MR. In the meantime, data such as those provided in the study by von Bardeleben et al13 are needed to inform us on the real-world experience and outcomes with the use of the MitraClip device.
Conflict of interest statement
A. Latib is a consultant for Medtronic, Abbott Vascular, Edwards Lifesciences and Cardiovalve. The other authors have no conflicts of interest to declare.

