
Abstract
Aims: The aim of this study was to identify the impact of previous aortic valve replacement (AVR) in MitraClip (MC) patients.
Methods and results: Data from the German transcatheter mitral valve interventions (TRAMI) registry were analysed in the light of previous AVR by means of either standard AVR (SAVR) or transcatheter AVR (TAVR). Out of 791 MC patients, 68 (8.6%) had been submitted to AVR (68.4% SAVR and 31.6% TAVR). The AVR group was significantly older (77.2±8.0 years vs. 75.1±8.6 years; p<0.05) and had a trend towards a higher risk profile (median STS score 10 [8.0-12.0] vs. 6.0 [3.0-11.0]; p=0.1). No procedural mortality was observed. Severe residual MV regurgitation was reported in 6.2% of AVR vs. 3.7% of the no-AVR patients (p=0.1). Thirty-day mortality was 10.6% in the previous AVR group vs. 3.9% in the no-AVR group (p<0.05). One-year estimated survival was lower in the AVR group (AVR 63% vs. no-AVR 81%; p<0.0001; HR 2.25, 95% CI: 1.42-3.55). Estimated survival in TAVR compared to SAVR was lower (TAVR 44.4% vs. SAVR 70%; p=0.039; HR 2.32, 95% CI: 0.99-5.37). AVR was a determinant of follow-up mortality (HR 2.18, 95% CI: 1.4-3.4; p<0.001).
Conclusions: Previous AVR in patients undergoing MC therapy carries a heavy and independent burden of mortality/morbidity.
Introduction
Aortic valve (AV) pathology and mitral regurgitation (MR) often coexist. As a result, 48% to 90% of candidates for either standard aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) for severe AV stenosis (AVS) present with some degree of MR at the time of AV treatment1,2. In up to 60% of these patients, a significant improvement of MR will be observed as a result of an increase in systolic left ventricular function after a sole AVR3. In those with worsening symptomatic MR, a subsequent intervention is often proposed in due course. In the light of their complex comorbid profile, and their previous AVR, these patients may be considered as candidates for minimally invasive forms of treatment of their MR.
In this context, the topic of previous AVR in patients undergoing percutaneous management of MR by means of MitraClip® (MC) (Abbott Vascular, Santa Clara, CA, USA) therapy has never been specifically addressed. The present study was developed within the premises of the independent German transcatheter mitral valve interventions (TRAMI) registry and discusses, in a “real-world” contemporary scenario, the occurrence of previous AVR (either SAVR or TAVR) in patients referred for MC therapy. Here we describe the demographic, clinical, and pathophysiology profile of such patients, we present their acute and midterm results after MC therapy, and we investigate if and how previous AVR may have an independent impact upon outcomes.
Methods
The non-randomised TRAMI registry was established in 2010 in order to assess the safety and efficacy of catheter-based mitral valve (MV) interventional techniques. The TRAMI registry has been supported by the “Stiftung Institut für Herzinfarktforschung” (Stiftung IHF) and an unrestricted grant from Abbott Vascular, Germany and “Deutsche Herzstiftung”.
Registry structure, timing, and analyses of results have been presented in previous publications, including acute and one-year follow-up findings4-6.
All patients included in the present TRAMI series had undergone MC implantation to treat symptomatic MR. No other forms of percutaneous treatment of MR were adopted. The following analysis includes patients who were prospectively enrolled into TRAMI and who were available for one-year follow-up. Data were prospectively collected in the TRAMI registry and retrospectively analysed.
All patients gave written informed consent and data were collected via web-based electronic case report forms4-6. Assessment of mitral regurgitation, device, and procedure success/failure was performed as previously described4-6. Major adverse cardiac and cerebrovascular events (MACCE) included death from any cause, stroke, and myocardial infarction.
Two groups were defined: those who had previously undergone AVR (AVR group) before being submitted to MC therapy and the remaining patients (no-AVR group). Subgroups for patients submitted to either TAVR or SAVR were identified within the AVR group.
STATISTICAL ANALYSIS
Categorical variables are presented as absolute numbers and percentages and are compared by the chi² test. Continuous variables are expressed as means with standard deviations or medians with interquartile ranges and are compared by the Mann-Whitney-Wilcoxon test. The cumulative one-year incidence of mortality was estimated by the Kaplan-Meier method with log-rank testing.
Multivariable Cox regression using forward selection was performed to identify the impact of previous AVR upon one-year mortality. We included all variables correlated with one-year mortality at a p-value <0.1 or expected to influence outcome from previous publications. Variables included in the Cox regression model were: female gender, age >75 years, previous AVR, NYHA Class IV at admission, sinus rhythm at admission, number of implanted clips ≥2, anaemia at admission, serum creatinine ≥1.5 mg/dL at admission, peripheral artery disease, prior stroke, lung diseases, prior cardiac decompensation, left ventricular ejection fraction <30%, severe tricuspid regurgitation, and procedural failure.
All tests were two-tailed and p-values <0.05 were considered significant. SAS statistical package version 9.3 (SAS Institute, Cary, NC, USA) was used for the computations.
Results
BASELINE CHARACTERISTICS
Between January 2010 and July 2013, 828 patients with symptomatic MR referred for MC therapy were enrolled prospectively in the TRAMI registry. In 37 patients, information about previous AVR was not given. Out of the remaining 791 patients, 68 (8.6%) had already been submitted to AVR (AVR group) for either AV stenosis or regurgitation (aetiology not clarified in the registry) and 723 (91.4%) patients had no previous AV treatment (no-AVR group). SAVR had been performed in 39 (68.4%) and TAVR in 18 (31.6%) patients (in 11 cases information concerning the technique adopted for AVR was missing).
The preprocedural characteristics of the two groups are reported in Table 1. Patients in the AVR group were significantly older (AVR group 77.2±8.0 years vs. no-AVR group 75.1±8.6 years; p<0.05) and had a higher incidence of lung disease (AVR group 45.6% vs. no-AVR group 30.2%; p<0.01). As a result, there was a non-significant trend towards a higher risk profile including higher STS score (median 10 vs. 6.0; p=0.13) and logistic EuroSCORE (median 24.0 vs. 20.0; p=0.10) in the AVR group (Table 1).
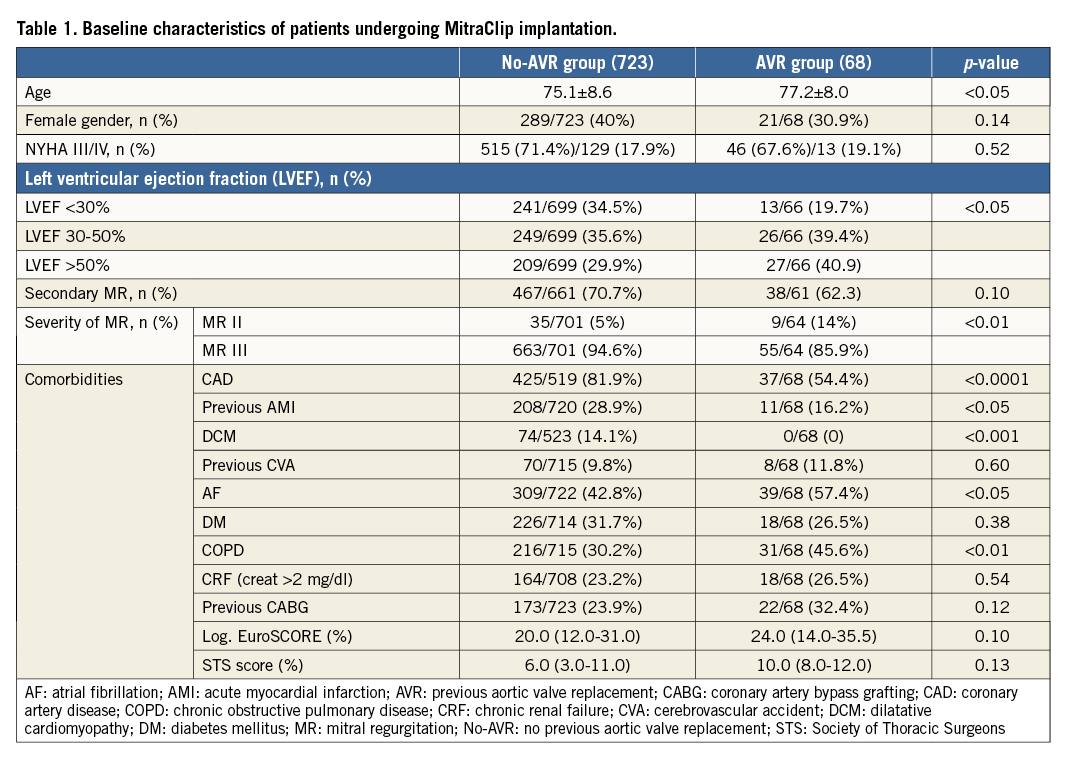
Patients in the no-AVR group had a significantly higher rate of cardiac comorbidities including previous diagnosis of coronary artery disease (CAD) (AVR group 54.4% vs. no-AVR group 81.9%, p<0.0001), previous acute myocardial infarction (AMI) (AVR group 16.2% vs. no-AVR group 28.9%, p<0.05), depressed left ventricular ejection fraction (LVEF) <30% (AVR group 19.7% vs. no-AVR group 34.5%, p<0.05), and dilatative cardiomyopathy (DCM) (AVR group 0 vs. no-AVR group 14.1%, p<0.0001) (Table 1).
Although the majority of patients in both groups were in NYHA Class III-IV and most of them were affected with secondary MR, severe MR at the time of referral for MC was significantly more common in the no-AVR group (AVR group 85.9% vs. no-AVR group 94.6%; p<0.01) (Table 1).
A sub-analysis between the TAVR and SAVR groups confirmed that TAVR candidates were significantly older (TAVR 81.4±5.5 vs. SAVR 76.4±8.4 years; p<0.05) and had a slightly more complex comorbid profile, resulting in a trend towards higher surgical risk (median logistic EuroSCORE: TAVR 27 vs. SAVR 24.5; p=0.71).
PERIPROCEDURAL, IN-HOSPITAL, AND 30-DAY OUTCOMES
No patient required intraprocedural conversion to conventional surgery and no intraoperative mortality was reported. Operation duration as well as average number of implanted MCs was similar in the two groups (Table 2).
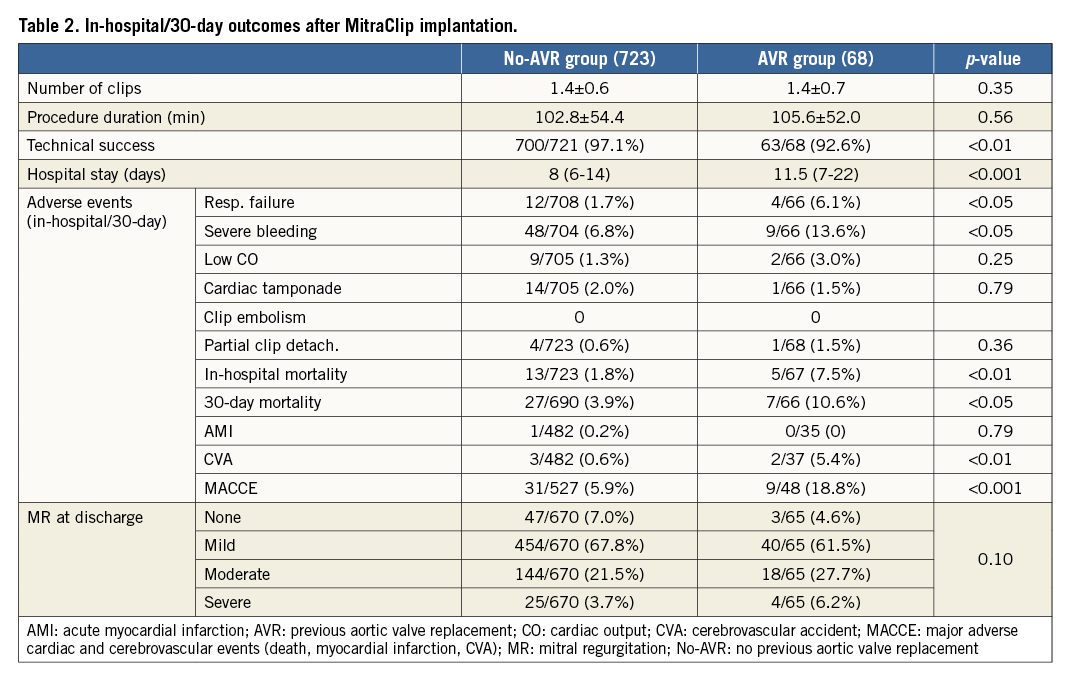
Technical success was achieved in 92.6% of the AVR group and 97.1% of the no-AVR group (p<0.01) (Table 2) (measured at exit from the catheterisation laboratory and including: absence of procedural mortality; successful access, delivery, and retrieval of the device delivery system; successful deployment and correct positioning of the first intended device; freedom from emergency surgery or reintervention related to the device or access procedure). More than grade II MR was reported at the end of the MC procedure in 8.8% of the AVR group and 2.1% of the no-AVR group (p<0.01).
Complications, such as requirement for blood transfusions, acute cerebrovascular accident, and prolonged mechanical ventilation secondary to post-procedural respiratory failure, were significantly more common in the AVR group and led to significantly higher in-hospital/30-day mortality (Table 2). Hospitalisation was significantly longer in the AVR group (p<0.001), and residual severe MV regurgitation at discharge was reported in 6.2% of the AVR group vs. 3.7% of the no-AVR group (p=0.10) (Table 2). In-hospital mortality was 7.5% in the AVR group and 1.8% in the no-AVR group (p<0.01).
Cumulative 30-day mortality and MACCE (mortality, myocardial infarction, and cerebrovascular accidents) were 10.6%/18.8% in the AVR group and 3.9%/5.9% in the no-AVR group (p<0.05 and p<0.001) (Table 2).
ONE-YEAR OUTCOMES
One-year follow-up was completed for 95% (59/62) of the surviving patients in the AV group and 90% (639/710) of the no-AVR group. Figure 1 depicts Kaplan-Meier survival curves for the no-AVR group and AVR group. One-year estimated survival was significantly lower in the AVR group (AVR 63% vs. no-AVR 81%; p<0.0001; HR 2.25; 95% CI: 1.42-3.55). A sub-analysis showed a significantly lower estimated survival in TAVR compared to SAVR patients (Figure 2) (TAVR 44.4% vs. SAVR 70%; p=0.039; HR 2.32; 95% CI: 0.99-5.37).
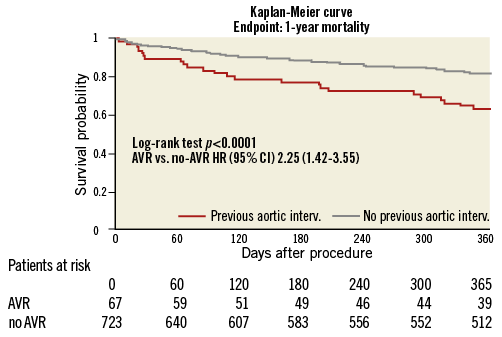
Figure 1. Kaplan-Meier survival curve in patients undergoing MC therapy with and without previous aortic valve replacement (AVR group and no-AVR group).
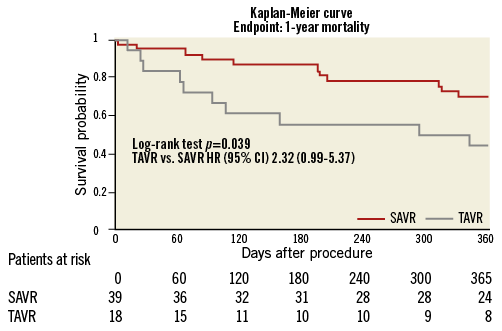
Figure 2. Kaplan-Meier survival curve in patients undergoing MC therapy after previous treatment with standard aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR).
Table 3 summarises one-year cumulative follow-up outcomes. Exact cumulative (hospital and post-hospital) mortality at one year was 37.5% in the AVR group and 18.9% in the no-AVR group (p<0.001). The cumulative endpoint (MACCE: death, myocardial infarction, cerebrovascular accidents) one-year rate was 50% in the AVR group and 24.6% in the no-AVR group (p<0.0001). During the one-year follow-up period, re-hospitalisation occurred in 78.4% of the AVR group and 62.7% of the no-AVR group (p=0.06). Causes for re-hospitalisation are summarised in Table 3. At one year, 48% of the surviving patients were in NYHA Class III-IV in the AVR group and 36.1% in the no-AVR group (p=0.23) (Table 3).
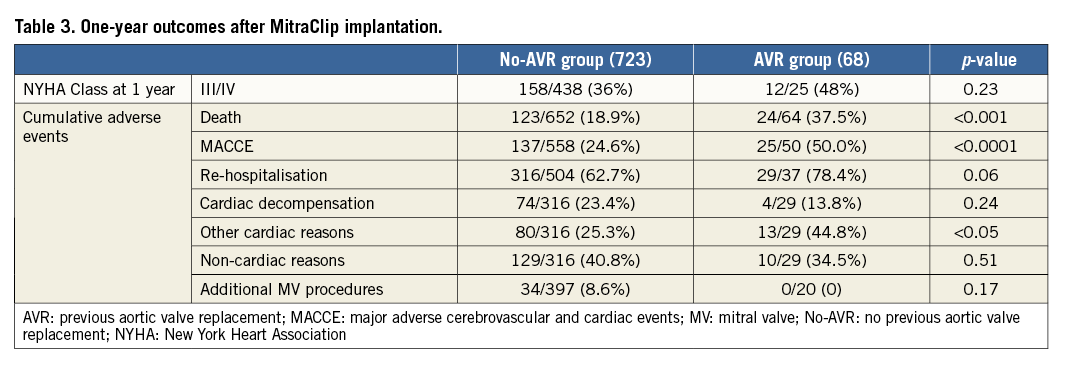
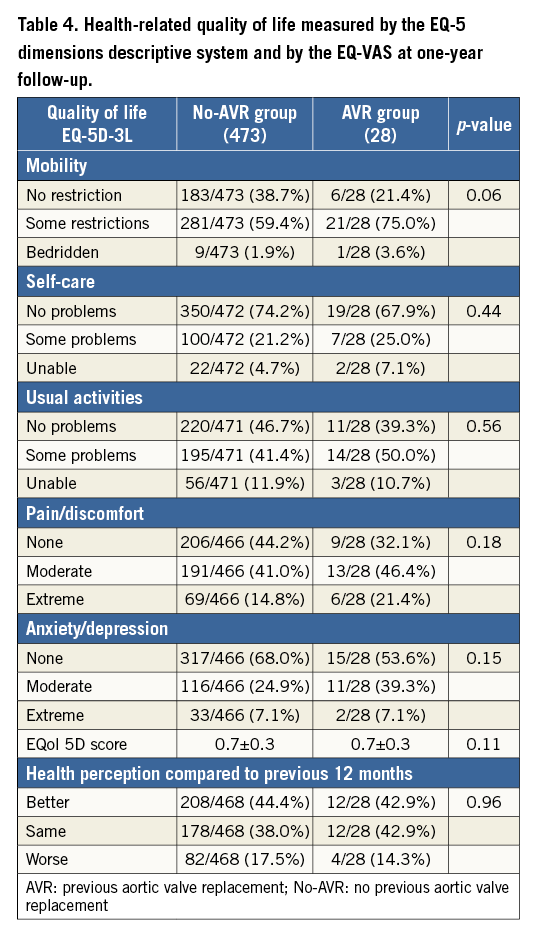
Health-related quality of life was measured by the EQ-5 dimensions descriptive system and by the EQ-VAS in 28 surviving patients in the AVR group and 473 in the no-AVR group. No significant differences were noticed in the follow-up evaluation of the surviving patients within the no-AVR group and AVR group (Table 4).
In order to quantify better the independent effect of previous AVR upon mortality (cumulative hospital and post-hospital mortality), a multivariable Cox regression model was built.
As already emphasised in a previous publication from the TRAMI registry6, previous AVR resulted in being an independent determinant for mortality (HR 2.18; 95% CI: 1.4-3.4; p<0.001) together with NYHA Class IV at admission (p=0.003), sinus rhythm (p=0.038), anaemia (p=0.012), serum creatinine ≥1.5 mg/dl (p=0.002), peripheral artery disease (p=0.001), LVEF <30% (p=0.008), severe tricuspid regurgitation (p=0.002), and procedural failure (p<0.0001).
Discussion
CARDIAC COMORBIDITIES AND MC
Patients referred to MC treatment carry a complex and heavy burden of comorbidities, as denoted in the data published within the contemporary registries and high-risk cohorts6-9. Within the plethora of preoperative risk factors, associated cardiac comorbidities may particularly aggravate the outcome after MC treatment, in the light of their additional and direct negative effect upon cardiac homeostasis and function. In reality, only a few authors have focused their attention upon the impact of cardiac comorbidities on the outcome of MC therapy. Within the premises of the TRAMI registry, Schwencke et al have recently discussed the effect of CAD, DCM, and AV disease upon acute and follow-up results of 528 TRAMI patients10. What emerges from this study is that patients with different cardiac comorbidities will share a similar burden of preoperative risks and, as a result, their perioperative and midterm outcomes will remain similar. In the light of their findings, the authors conclude that MC therapy is feasible and safe even in high-risk patients with associated cardiac comorbidities10.
PREVIOUS AVR AND MC
Although, as documented by Schwencke et al10, concomitant AV disease at the time of MC therapy does not seem to impair outcomes when compared with other cardiac comorbidities, the specific impact of previous treatment of AV disease by means of AVR may be more marked.
In the present manuscript, we have tried to elucidate if and eventually in which terms previous AVR will impact upon MC therapy outcome. In this context, some key messages emerge.
The actual rate of previous AVR in patients undergoing surgical MV repair is scarcely represented in the existing literature. From a recent report of the STS, it emerges that, out of over 15,000 patients undergoing mitral valve repair, just 3.6% had already had some sort of previous valve surgery (not specified if aortic or mitral)11. In our TRAMI data, 8.6% of patients undergoing MC therapy had already undergone AVR (either SAVR or TAVR). This finding confirms the fact that after AVR an increasing number of patients are nowadays referred to percutaneous treatment of their MR instead of conventional MV surgery, most probably in the light of their complex comorbid profile.
What emerges from our data is that, even in the very early phases of MC therapy, AVR patients will perform significantly worse. In particular, technical success of MC therapy is significantly less common in the AVR group where a larger number of patients will be discharged with moderate and severe residual MR. This finding is in line with the analysis of Schwencke et al where residual moderate MR was more often present in AV disease patients when compared to patients with CAD and DCM10.
The worse outcome in the AVR group could derive from: a) a different pathophysiology of MR, and b) a more complex preprocedural comorbid profile. In fact, patients in the AVR group are significantly older and, in the ageing population, multiple valve disease is not uncommon, being caused mainly by calcific degeneration of the AV and MV12. As confirmed by our findings, AVR patients are more often referred to MC therapy for primary (degenerative) MR and less often have associated cardiac comorbidities such as impaired LVEF, DCM, and CAD that would usually lead to secondary MR. Treatment of degenerative (primary) MR by means of MC may result in being more challenging when compared to functional (secondary) MR. In fact, clipping of calcific and degenerated leaflets may be technically more demanding13. This anatomical preprocedural condition may have impacted upon procedural success.
Apart from a lower technical success rate, patients in the AVR group experience a higher in-hospital and 30-day mortality. This could be partly explained by the clinical complexity of the AVR patients that, as already emphasised, includes advanced age and higher rates of comorbidities such as COPD, atrial fibrillation, and of course the presence of a previously implanted prosthetic AV. All these conditions favour a more troubled periprocedural course, including significantly higher rates of respiratory failure, profuse bleeding, thromboembolism, and CVAs that eventually result in significantly elongating the required hospitalisation and increasing the in-hospital mortality.
Understanding how previous AVR will impact upon follow-up outcomes after MC therapy may be of interest in order to optimise the everyday patient selection strategy. In a recent analysis of the TRAMI registry, Puls et al were the first to define the influence of previous AVR upon midterm outcomes of patients referred for MC therapy. Among the numerous preprocedural comorbidities, previous AVR at the time of MC therapy was the strongest independent determinant of one-year mortality6.
Our present analysis, including a slightly larger number of TRAMI patients, has confirmed that, independently from any other preprocedural demographic/clinical variable, previous AVR more than doubles the risk of midterm mortality after MC therapy (HR 2.18; 95% CI: 1.4-3.4; p<0.001). As a result, midterm observed mortality in these patients is almost 40%, the highest ever found after MC therapy.
Apart from an increased perioperative and midterm mortality after MC therapy, the AVR group has, in our experience, a heavy toll of one-year morbidity and re-hospitalisation. In fact, additional major adverse events occur in one out of two patients in the AVR group and will result in an almost 80% one-year re-hospitalisation rate after MC therapy. Interestingly, the majority of hospital readmissions in the AVR group are not due to cardiac decompensation but to additional cardiac reasons, not specified in the TRAMI data set, and that could be, at least in theory, ascribed to the presence of an aortic prosthesis.
Quality of life and clinical performance after MC therapy are also important to define the effective benefits of such a treatment. Apart from significantly increased major events and re-hospitalisation rates, surviving patients in the AVR group have a trend towards a worse overall physical health after MC therapy. In fact, almost one in two patients will remain in NYHA Class III/IV with some perceived problems in performing their usual daily activities.
TAVR AND MC
Specific comments should be made about patients undergoing MC therapy after being previously submitted to percutaneous AV interventions. TAVR has been popularised as a safe and effective alternative form of treatment in patients with severe AVS who have an unacceptably high surgical risk. In two of the largest prospective randomised trials comparing TAVR to SAVR, TAVR has shown a one-year mortality rate below 25% with a MACCE rate of around 30% and a re-hospitalisation rate below 20%14,15. Results may differ when focusing upon patients with an associated MV pathology. Although we do not know how many patients in the TRAMI registry already presented some sort of MV malfunction at the time of referral for TAVR and how much time passed between TAVR and MC therapy, there is evidence that patients with moderate or severe MR undergoing TAVR exhibit a worse baseline clinical profile and have higher one-year mortality. In a recent meta-analysis of eight studies involving 8,927 patients, Chakravarty et al evaluated the impact of MR on outcomes after TAVR3. The presence of moderate-severe MR at baseline was associated with increased mortality at thirty days and one year. Moreover, while the severity of MR improved in approximately 60% of patients after sole TAVR, residual moderate-severe MR was associated with significantly increased one-year mortality after TAVR3. Although this subgroup of patients with persistent severe MR is often proposed for MC therapy, outcomes should be analysed critically, in the light of the tremendous additional clinical and budgetary burden involved. In fact, from our reported experience in TAVR patients undergoing MC therapy, the estimated one-year survival of these patients is well below 50%. In the light of these findings within the TRAMI pool of patients, the real benefits of an additional percutaneous MV treatment in patients previously submitted to TAVR should be investigated in a prospective fashion and compared to maximal medical treatment.
Limitations
The present manuscript is based on a national registry and has all the consequent limitations. In particular, data are self-reported and information is at times incomplete. Furthermore, echocardiographic data were not core-lab adjudicated and therefore of minor quality. Follow-up at thirty days and at one year was performed by telephone call and therefore does not include an echocardiography. Finally, the time duration between AVR and MC therapy was not included in the manuscript because it is not available in the TRAMI registry.
In any case, although prospective randomised trials are nowadays used to support medical decisions and draw up guidelines, they often do not represent the “real world” of patients who are referred to medical practitioners. Registries such as TRAMI mirror the daily pattern of referrals, treatments, and outcomes.
Conclusions
As shown in our data, previous AVR has already occurred in approximately 10% of patients referred nowadays to MC therapy for severe symptomatic MR.
Although MC therapy after AVR is feasible, a heavier burden of technical failure, in-hospital and midterm mortality/morbidity is expected together with a high re-hospitalisation rate for cardiovascular reasons. All these findings are only partly explained by the patients’ overall comorbid profile. In fact, previous AVR is clearly an independent determinant for worse outcome at follow-up.
| Impact on daily practice Professionals involved in the percutaneous treatment of structural heart disease will observe an increase in the referral of patients with double valve pathology. In particular, the rate of previous AVR in MC candidates may reach 10%, and this particular subgroup of patients represents a real clinical and technical challenge, in the light of its complex comorbid profile and valve physiopathology. As a result, worse acute and follow-up outcomes with adverse events and re-hospitalisation over 50% are expected and should be disclosed. |
Funding
The TRAMI registry has been supported by “Stiftung Institut für Herzinfarktforschung” (Stiftung IHF) and an unrestricted grant from Abbott Vascular, Germany and “Deutsche Herzstiftung”.
Conflict of interest statement
H. Ince, W. Schillinger and H. Sievert have received personal fees from Abbott Vascular. M. Puls and W. Schillinger have received travel expenses from Abbott Vascular. The other authors have no conflicts of interest to declare.

