Abstract
Background: Concomitant moderate/severe mitral regurgitation (MR) is observed in 17-35% of patients undergoing transcatheter aortic valve implantation (TAVI) and contributes to a worse prognosis. Studies analysing outcomes in patients undergoing TAVI with different MR aetiologies, including atrial functional MR (aFMR), are lacking.
Aims: We aimed to analyse outcomes and changes in MR severity in patients with aFMR, ventricular functional (vFMR) and primary mitral regurgitation (PMR) following TAVI.
Methods: We analysed all consecutive patients with at least moderate MR undergoing TAVI between January 2013 and December 2020 at the Munich University Hospital. Characterisation of MR aetiology was performed by detailed individual echocardiographic assessment. Three-year mortality, changes in MR severity and New York Heart Association (NYHA) Functional Class at follow-up were assessed.
Results: Out of 3,474 patients undergoing TAVI, 631 patients showed MR ≥2+ (172 with aFMR, 296 with vFMR, 163 with PMR). Procedural characteristics and endpoints were comparable between groups. The rate of MR improvement was 80.2% in aFMR patients, which was significantly higher compared to both other groups (vFMR: 69.4%; p=0.03; PMR: 40.8%; p<0.001). The estimated 3-year survival rates did not differ between aetiologies (p=0.57). However, MR persistence at follow-up was associated with increased mortality (hazard ratio 1.49, 95% confidence interval: 1.04-2.11; p=0.027), mainly driven by the PMR subgroup of patients. NYHA Class improved significantly in all groups. In patients with baseline MR ≥3+, the PMR aetiology was associated with the lowest MR improvement, the lowest survival rates and least symptomatic improvement.
Conclusions: TAVI reduces MR severity and symptoms in patients with aFMR, vFMR and less-pronounced PMR. The presence of aFMR was associated with the greatest MR severity improvement.
Introduction
Transcatheter aortic valve implantation (TAVI) is currently the treatment of choice for elderly patients with symptomatic severe aortic stenosis (AS) at high surgical risk12 and is increasingly used in patients at lower surgical risk34. Concomitant moderate and severe mitral regurgitation (MR) is observed in 17-35% of patients undergoing TAVI15678 and is associated with a worse prognosis89. The decrease in afterload due to the elimination of outflow obstruction after TAVI has been shown to reduce MR severity in 50-60% of patients with functional (FMR) and primary MR (PMR)6710.
While FMR often originates from pathological alterations in left ventricular systolic function and geometry11 (ventricular FMR [vFMR]), some patients show normal left ventricular dimensions and systolic function while suffering from severe heart failure (HF) symptoms due to significant MR121314. This phenotype, named atrial functional MR (aFMR), is frequently related to atrial fibrillation, diastolic dysfunction, and HF with preserved ejection fraction (HFpEF), where atrial enlargement with subsequent dilatation of the mitral annulus leads to MR development131516. While larger studies analysing the outcomes of TAVI-treated patients with concomitant MR of different aetiologies are rare, the unique pathophysiology of aFMR in this context is underrecognised, and data on this topic are scarce.
To address this gap in knowledge, we aimed to analyse mortality, outcome predictors, long-term MR severity development and the symptomatic improvement of patients with aFMR compared to vFMR and PMR patients following TAVI.
Methods
Study population
All consecutive patients undergoing transfemoral TAVI between January 2013 and December 2020 at Munich University Hospital (Munich, Germany) with available baseline MR information were included in this analysis. For the purpose of this study, all patients showing MR ≥2+ were screened for categorisation into the aFMR, vFMR and PMR groups. Patients with prior mitral valve intervention or mitral valve surgery were excluded from this analysis.
Before TAVI, a multidisciplinary Heart Team consensus by interventional cardiologists and cardiac surgeons was obligatory to evaluate the best treatment option in each individual patient. Patient data were collected and stored in a database according to the local requirements for quality control, in accordance with the Declaration of Helsinki. Ethical approval was obtained from the institutional ethics board (EVERY-Valve-Registry, ethical code number 19-840; date: 20 December 2019). Clinical and echocardiographic follow-up information was obtained either by phone, during hospital admissions, or at outpatient clinic visits, as previously described17.
Echocardiography
Transthoracic echocardiographic images were obtained prior to the TAVI procedure, in accordance with current European and American guidelines1819. The severity of AS was assessed using the continuity equation method. Before discharge, valve function, including the presence of paravalvular leaks, was evaluated as suggested by the recently published Valve Academic Research Consortium 3 (VARC-3) guidelines20. Baseline MR severity was assessed according to the current recommendations of the American Society of Echocardiography21. The mitral annular anteroposterior (AP) diameter was measured in a 4-chamber view at the time of end-systole/early diastole. Left atrial (LA) dilation was defined as an indexed LA volume of >34 ml/m2, as previously described122223. Each preprocedural transthoracic echocardiography was individually assessed by an experienced physician to ensure a precise characterisation of aetiology without interobserver variability. In the case of mixed aetiologies, the leading aetiology was respected. Patients were considered to have aFMR when showing preserved left ventricular ejection fraction (LVEF) (i.e., HFpEF), LVEF ≥50%, with normal indexed left ventricular (LV) body surface area dimensions and without any regional wall motion abnormalities, a Carpentier type I leaflet motion1224, dilated left atria (>34 ml/m2), and absence of leaflet calcifications. Patients with reduced left ventricular ejection fraction, regional wall motion abnormalities, abnormal shape or increased left ventricular dimensions, were considered to have vFMR. Patients showing predominantly preserved LV function and dimensions with mitral valvular leaflet calcifications, damage or prolapse/flail were considered to have PMR. For echocardiographic follow-up information regarding MR severity, images were retrospectively analysed where available. In cases where images were not available, written reports were used. For the analysis of MR improvement, only patients with complete follow-up information were included.
TAVI procedure
All procedures were performed under local anaesthesia. Transfemoral access was used in all patients. Preprocedural anticoagulation was achieved with unfractionated heparin (50 to 70 IU/kg body weight). The decision to perform pre- and/or post-dilation was left to the operator’s discretion. For access-site haemostasis, suture-mediated closure devices were used. Antithrombotic therapy consisted of dual antiplatelet therapy with 100 mg aspirin and 75 mg clopidogrel for 3 months, followed by 100 mg aspirin lifelong in patients without concomitant percutaneous coronary intervention (PCI). In patients with an indication for oral anticoagulation, therapy was continued after the TAVI procedure. In patients undergoing PCI, antiplatelet and anticoagulation regimens were conducted according to current guidelines25.
Trial endpoints and follow-up
In this analysis, the primary aim was to assess long-term changes in MR severity and its impact on 3-year mortality within the different MR aetiologies. MR persistence was defined as no change or worsening of MR severity at last available follow-up. MR improvement was defined as an improvement of MR severity of at least 1 grade. As secondary outcomes, we assessed procedural endpoints according to the new 2021 VARC-3 criteria20 and heart failure symptoms, defined by New York Heart Association (NYHA) Functional Class. In case of a mitral valve intervention or surgery during follow-up, the last echocardiographically measured MR grade before intervention was used, and patients were considered to have persistent MR.
Statistical analysis
For descriptive statistics, continuous data are presented as means with standard deviation (SD) or medians with interquartile ranges (IQR), respectively. Categorical data are presented as proportions. The normality of data distribution was assessed graphically and using the Shapiro-Wilk test. Comparisons between groups were performed using Pearson’s Chi-squared test for categorical variables, the Student’s t-test or Mann-Whitney U test for unpaired continuous variables and the Wilcoxon rank-sum test for paired variables, according to data distribution. Cumulative survival after 3 years was estimated and graphically displayed using Kaplan-Meier curves. Predictors for MR persistence (MR ≥3+) were assessed with binomial logistic regression. Candidate predictors with a level of significance of <0.05 were considered in the multivariable regression analysis. Results are expressed as hazard ratios (HR) or odds ratios (OR) with 95% confidence intervals (CI).
A p-value of <0.05 was regarded as statistically significant. The statistical software used for data analysis and visualisation was R version 1.4.1717 (The R Foundation for Statistical Computing).
Results
Study sample and clinical characteristics of MR subtypes
Out of 3,474 patients that underwent TAVI at Munich University Hospital, information on baseline MR was available in 3,151 patients. A study flowchart is shown in Supplementary Figure 1. Baseline characteristics of those patients are shown in Supplementary Table 1. The mean follow-up was 1.88±1.22 years. Mortality was significantly higher in patients with moderate or more than moderate MR compared to patients with no or mild MR (HR 1.40, 95% CI: 1.21-1.62 and HR 2.10, 95% CI: 1.70-2.59; p<0.001 for both) (Figure 1).
A total of 631 patients (median age of 83 [77.8, 86.7] years) had MR ≥2+ prior to TAVI (Central illustration, Supplementary Figure 1). All baseline clinical and echocardiographic characteristics are presented in Table 1 and Table 2.
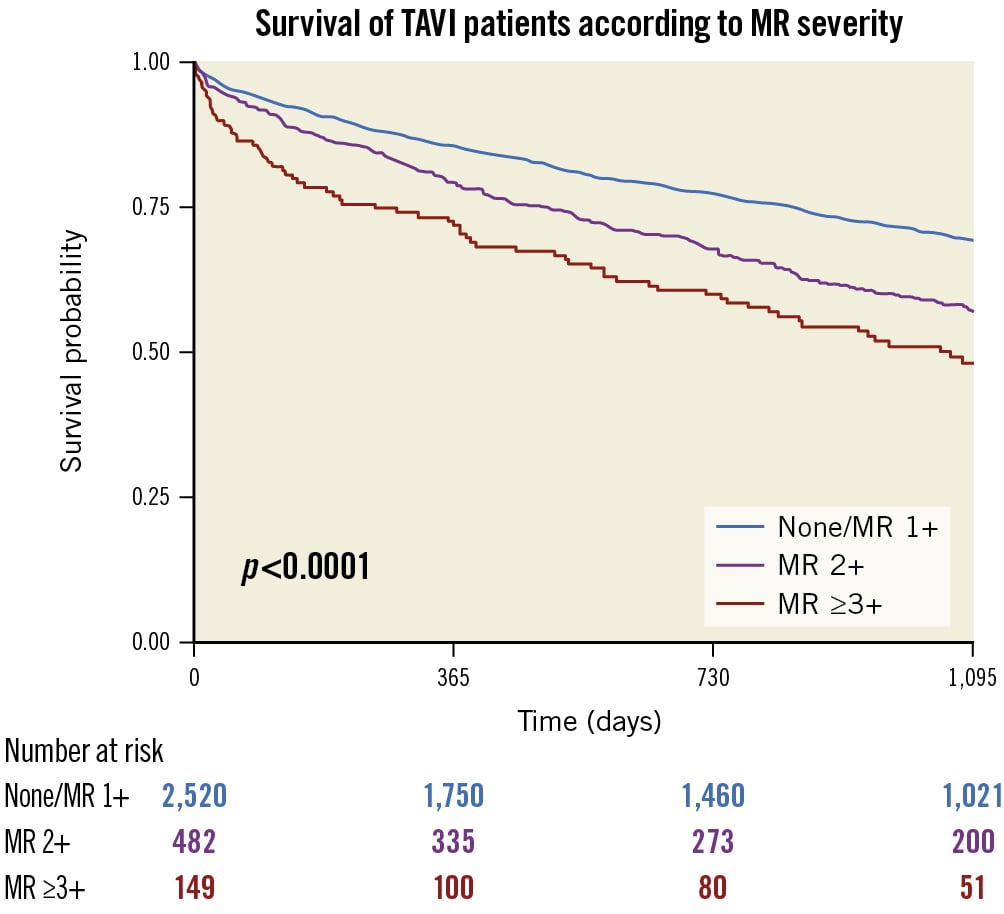
Figure 1. Survival of TAVI patients according to MR severity. This figure shows the 3-year survival of patients according to their MR baseline severity. MR: mitral regurgitation; TAVI: transcatheter aortic valve implantation
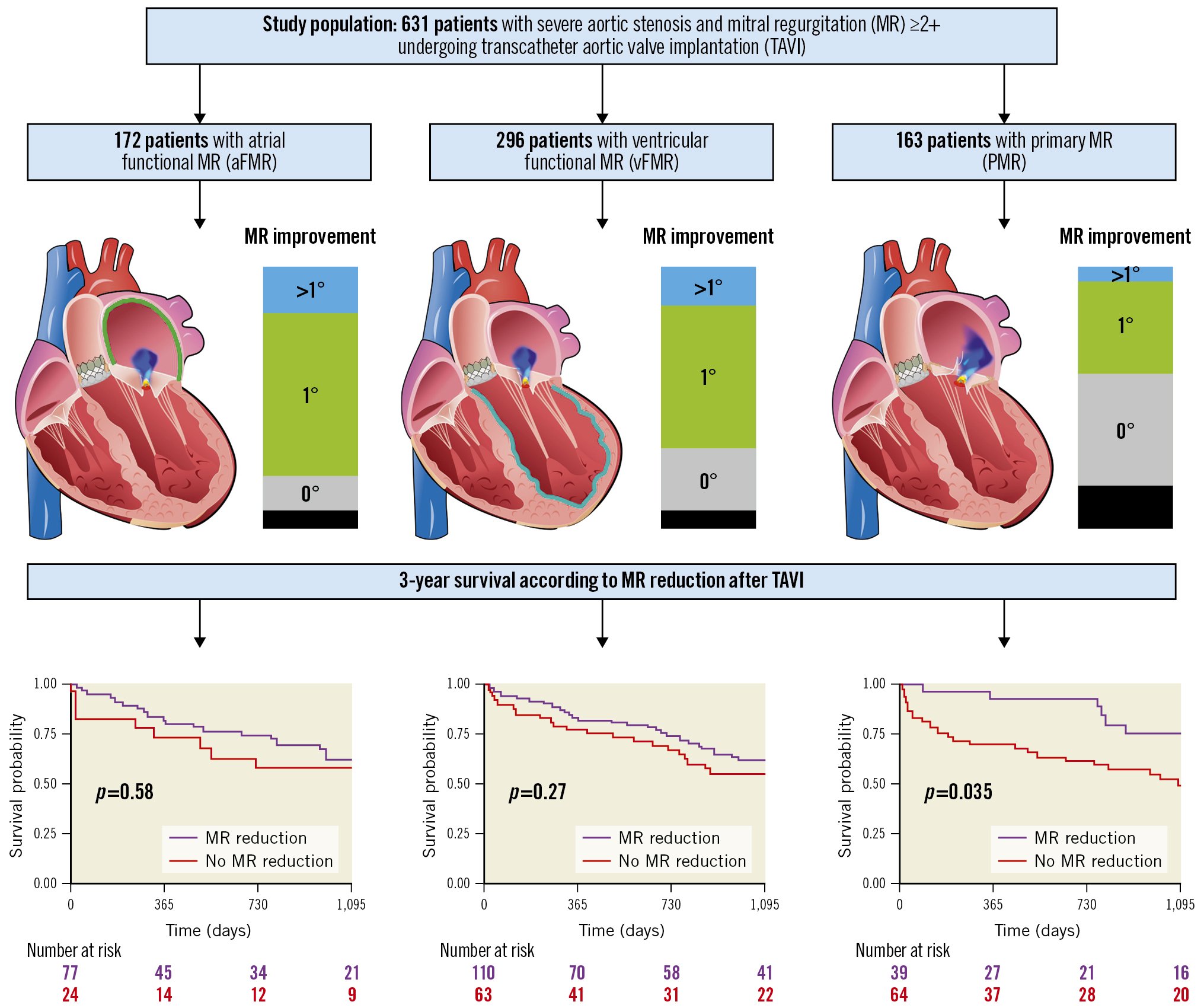
Central illustration. The three MR aetiologies of the study population, MR improvement and 3-year survival. This illustration demonstrates the three MR aetiologies aFMR, vFMR and PMR, including MR improvement and 3-year survival.
Table 1. Baseline characteristics of patients.
| Overall | aFMR | vFMR | PMR | p-value | ||
|---|---|---|---|---|---|---|
| n | 631 | 172 | 296 | 163 | ||
| Age, years | 82.7 [77.8, 86.7] | 83.1 [78.5, 86.8] | 82.0 [77.2, 86.7] | 83.7 [79.0, 86.2] | 0.16 | |
| Sex (female) | 331 (52.0) | 99 (57.6) | 118 (39.9) | 109 (66.9) | <0.01 | |
| BMI, kg/m2 | 24.6 [22.4, 27.6] | 24.7 [22.6, 27.8] | 24.6 [22.1, 27.4] | 24.6 [22.3, 27.4] | 0.85 | |
| STS score | 5.0 [3.0, 7.5] | 4.0 [2.9, 7.0] | 5.0 [3.0, 7.8] | 5.0 [3.1, 8.1] | 0.12 | |
| eGFR, ml/min | 46.5±27.1 | 48.1±19.2 | 45.2±21.8 | 47.0±24.0 | 0.38 | |
| NYHA Functional Class | I | 7 (1.1) | 0 (0.0) | 4 (1.4) | 3 (1.9) | <0.01 |
| II | 50 (8.2) | 18 (10.9) | 13 (4.6) | 18 (11.5) | ||
| III | 437 (71.5) | 125 (75.8) | 196 (68.8) | 112 (71.8) | ||
| IV | 112 (18.3) | 22 (13.3) | 68 (23.9) | 22 (14.1) | ||
| Hyperlipidaemia | 260 (42.8) | 73 (44.5) | 124 (43.2) | 63 (40.4) | 0.75 | |
| Hypertension | 540 (88.7) | 148 (89.7) | 253 (87.8) | 139 (89.1) | 0.82 | |
| Smoking | 119 (19.8) | 27 (16.8) | 71 (24.9) | 21 (13.5) | 0.01 | |
| Diabetes | 179 (29.3) | 40 (24.2) | 82 (28.5) | 57 (36.3) | 0.05 | |
| Positive family history of cardiovascular events | 59 (9.9) | 12 (7.5) | 33 (11.7) | 14 (9.0) | 0.33 | |
| COPD | 101 (16.1) | 34 (19.9) | 42 (14.3) | 25 (15.3) | 0.27 | |
| Coronary artery disease | 382 (62.6) | 89 (53.9) | 195 (67.7) | 98 (62.4) | 0.01 | |
| Previous MI | 112 (18.9) | 14 (8.6) | 74 (26.5) | 24 (15.8) | <0.01 | |
| Previous PCI | 209 (34.6) | 44 (26.7) | 115 (40.6) | 50 (32.1) | 0.01 | |
| Previous CABG | 59 (9.8) | 12 (7.3) | 34 (12.0) | 13 (8.3) | 0.21 | |
| Atrial fibrillation | 329 (51.7) | 122 (70.9) | 132 (44.6) | 72 (44.2) | <0.01 | |
| Prior biological AV prosthesis | 52 (8.2) | 12 (7.0) | 30 (10.1) | 10 (6.1) | 0.26 | |
| Prior cardiac surgery | 103 (17.0) | 23 (14.0) | 58 (20.2) | 22 (14.1) | 0.13 | |
| Qualitative data are presented as n (%); Quantitative data are presented as median [IQR] or mean±standard deviation. aFMR: atrial functional mitral regurgitation; AV: aortic valve; BMI: body mass index; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; MI: myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PMR: primary mitral regurgitation; STS: Society of Thoracic Surgeons; vFMR: ventricular functional mitral regurgitation | ||||||
Table 2. Baseline echocardiographic characteristics of patients.
| Overall | aFMR | vFMR | PMR | p-value | ||
|---|---|---|---|---|---|---|
| n | 631 | 172 | 296 | 163 | ||
| LVEF, % | 50.0 [40.0, 55.0] | 55.0 [54.8, 58.2] | 40.0 [35.0, 47.0] | 55.0 [50.0, 58.0] | <0.01 | |
| LVEDD, cm | 4.7 [4.0, 5.3] | 4.3 [3.9, 4.9] | 5.0 [4.5, 5.6] | 4.5 [3.8, 5.2] | <0.01 | |
| LVESD, cm | 3.5 [3.0, 4.2] | 3.1 [2.8, 3.5] | 4.0 [3.4, 4.6] | 3.3 [2.7, 4.0] | <0.01 | |
| LVEDV index, ml/m² | 59.1 [45.5, 76.2] | 49.2 [41.3, 60.1] | 70.1 [54.9, 88.7] | 55.1 [40.2, 68.1] | <0.01 | |
| LAV index, ml/m² | 52.4 [40.7, 70.2] | 55.1 [43.9, 73.0] | 50.1 [37.7, 65.2] | 53.6 [42.3, 73.9] | <0.01 | |
| AV dpmean, mmHg | 33.0 [24.0, 43.0] | 38.5 [28.0, 48.0] | 30.0 [21.0, 40.0] | 34.0 [25.0, 47.0] | <0.01 | |
| AV dpmax, mmHg | 54.0 [40.0, 70.0] | 65.0 [46.0, 79.0] | 49.0 [34.5, 63.0] | 54.0 [42.0, 73.2] | <0.01 | |
| AV opening area, cm² | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.9] | 0.7 [0.6, 0.9] | 0.7 [0.5, 0.8] | 0.12 | |
| SVI | 32.7 [25.5, 39.5] | 37.4 [31.3, 45.4] | 29.5 [22.8, 35.4] | 32.5 [26.1, 40.0] | <0.01 | |
| Mitral regurgitation grade | 2+ | 454 (71.9) | 133 (77.3) | 216 (73.0) | 105 (64.4) | 0.02 |
| 3+ | 142 (22.5) | 32 (18.6) | 68 (23.0) | 42 (25.8) | ||
| 4+ | 35 (5.5) | 7 (4.1) | 12 (4.1) | 16 (9.8) | ||
| Mitral annular diameter, mm | 32.0 [28.0, 36.0] | 31.0 [28.0, 34.5] | 32.0 [29.0, 36.0] | 31.0 [26.0, 36.0] | 0.03 | |
| Biplanar vena contracta, cm | 0.4 [0.3, 0.5] | 0.4 [0.2, 0.5] | 0.4 [0.3, 0.5] | 0.4 [0.3, 0.5] | 0.02 | |
| Tricuspid regurgitation grade* | 0 | 41 (7.0) | 14 (8.5) | 17 (6.4) | 10 (6.5) | 0.48 |
| 1+ | 310 (52.8) | 88 (53.3) | 146 (54.7) | 76 (49.0) | ||
| 2+ | 166 (28.3) | 50 (30.3) | 69 (25.8) | 47 (30.3) | ||
| 3+ | 49 (8.3) | 7 (4.2) | 28 (10.5) | 14 (9.0) | ||
| 4+ | 21 (3.6) | 6 (3.6) | 7 (2.6) | 8 (5.2) | ||
| RAV index, ml/m² | 39.5 [29.1, 55.1] | 39.0 [28.6, 53.5] | 41.1 [30.0, 55.8] | 37.6 [26.3, 54.6] | 0.19 | |
| RV midventricular diameter, mm | 31.0 [26.0, 37.0] | 31.0 [26.0, 35.0] | 33.0 [28.0, 38.0] | 29.0 [24.0, 36.0] | <0.01 | |
| TAPSE, mm | 18.0 [15.0, 22.0] | 20.0 [16.8, 23.0] | 17.0 [14.0, 21.0] | 19.0 [16.0, 23.0] | <0.01 | |
| RV/RA gradient, mmHg | 40.0 [31.0, 50.0] | 39.0 [30.0, 50.0] | 39.0 [30.0, 49.0] | 44.0 [34.0, 55.0] | 0.02 | |
| Qualitative data are presented as n (%); Quantitative data are presented as median [IQR]. *Data present for a total of 587 patients and data missing for 44 patients. aFMR: atrial functional mitral regurgitation; AV: aortic valve; dpmax: maximum pressure gradient; dpmean: mean pressure gradient; LAV: left atrial volume; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MR: mitral regurgitation; PMR: primary mitral regurgitation; RA: right atrium; RAV: right atrial volume; RV: right ventricle; SVI: stroke volume index; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid valve regurgitation; vFMR: ventricular functional mitral regurgitation | ||||||
Patients with functional MR
Out of the study sample of 631 patients, 172 were characterised as aFMR and 296 as vFMR (Central illustration) The clinical and echocardiographic characteristics differed considerably between aFMR and vFMR patients: aFMR patients were predominantly female (57.6% vs 39.9%; p<0.01); numerically older (83.1 years [78.5, 86.8] vs 82.0 years [77.2, 86.7]; p=0.16); and had a numerically lower median Society of Thoracic Surgeons (STS) score of (4.0 [2.9, 7.0] vs 5.0 [3.0, 7.8]; p=0.12) compared to vFMR patients. History of atrial fibrillation was more prevalent in aFMR than in vFMR patients (70.9% vs 44.6%; p<0.01). Compared to patients with aFMR, the percentage of patients with NYHA Class IV before TAVI was significantly higher in vFMR patients (vFMR: 23.9% vs aFMR: 13.3 %; p<0.01).
Baseline echocardiographic parameters differed considerably between groups. The median LVEF and left atrial volume index (LAV index) were significantly higher in aFMR compared to vFMR patients (LVEF: 55.0 [54.8, 58.2]% vs 40.0 [35.0, 47.0]%; p<0.01; LAV index: 55.1 [43.9, 73.0] ml/m2 vs 50.1 [37.7, 65.2] ml/m2); p<0.01. Both, the mean (dpmean) and maximum baseline aortic valve pressure gradients (dpmax) were lower in vFMR patients (dpmean: 30.0 [21.0, 40.0] mmHg vs 38.5 [28.0, 48.0] mmHg; p<0.01; dpmax: 49.0 [34.5, 63.0] mmHg vs 65.0 [46.0, 79.0] mmHg; p<0.01 for vFMR vs aFMR, respectively). vFMR patients showed larger mitral annular diameters (32.0 [29.0, 36.0] vs 31.0 [28.0, 34.5]; p=0.02) and the tricuspid annular plane systolic excursion value was significantly lower compared to aFMR patients (17.0 [14.0, 21.0] vs 20.0 [16.8, 23.0]; p<0.01). MR grades also differed significantly between groups. A detailed overview of all echocardiographic baseline characteristics is displayed in Table 2.
Patients with primary MR
Out of 631 patients with relevant MR, 163 were characterised as PMR (Central illustration). PMR patients were predominantly female (67%) and had comparable age and perioperative risk, with a median STS score of 5.0 [3.1, 8.1], as patients with aFMR and vFMR. Thirty-six percent of PMR patients had diabetes. The median LVEF was 55%, and 58 patients (35.6%) showed MR ≥3+ before the TAVI procedure. A detailed overview of all clinical and echocardiographic baseline characteristics is displayed in Table 1 and Table 2.
Procedural results
The majority of patients were treated with balloon-expandable valves (75%). Procedural characteristics were comparable between all groups and are presented in Supplementary Table 2. Technical failure and device failure at 30 days were comparable between patients with aFMR, vFMR and PMR. There was also no difference in the degree of paravalvular AR at the last available follow-up between groups (p=0.54). All procedural characteristics and endpoints according to VARC-3 are presented in Supplementary Table 2.
Mitral regurgitation grade after TAVI
The mean time to echocardiographic follow-up was 561±682 days, and there was no difference in time to echocardiographic follow-up between the three groups (p=0.40). Echocardiographic follow-up information was complete in 387 patients (61.8%). The follow-up rate did not differ between groups. During the follow-up period, 21 patients were treated with transcatheter edge-to-edge mitral valve repair after TAVI (3 patients [1.7%] in the aFMR group, 12 [4.1%] in the vFMR group and 6 [3.7%] in the PMR group). Two patients (1 aFMR patient, 1 PMR patient) received a surgical mitral valve replacement during the follow-up period. While MR severity significantly improved in all three groups after TAVI (Figure 2), among PMR patients, the rate of MR ≥3+ at follow-up (FU) was significantly higher compared to both other groups (MR ≥3+ at FU: PMR 33.0%, aFMR 4.7%, vFMR 8.0%; p<0.001 for both PMR vs aFMR and PMR vs vFMR) (Figure 2).
aFMR patients showed the highest rates of MR improvement of at least 1 grade, and PMR was associated with the lowest rate of MR improvement at follow-up (MR improvement: aFMR 80.2%; vFMR 69.4%; PMR 40.8%; p=0.03 for aFMR vs vFMR and p<0.001 for aFMR vs PMR) (Central illustration).
Considering all patients with MR ≥2+, MR improvement was associated with lower 3-year mortality (p=0.027 by log-rank test) (Supplementary Figure 2). However, this was not the case for both of the functional MR groups (p=0.58 and p=0.27, for aFMR and vFMR, respectively) (Central illustration). Overall increased mortality in patients with no MR improvement was driven by PMR patients, where 3-year mortality was significantly higher after TAVI (p=0.035) (Central illustration).
Besides, a multivariable logistic regression analysis revealed an increased left atrial volume index (OR 1.01, 95% CI: 1.00-1.02; p=0.01) and the presence of PMR as independent predictors for MR persistence following TAVI (PMR: OR 2.73, 95% CI: 1.60-4.80; p<0.001) (Table 3). The presence of aFMR was associated with a lower risk for MR persistence after TAVI (OR 0.50, 95% CI: 0.27-0.95; p=0.04) (Table 3). Of note, higher grades of baseline MR were not associated with MR persistence.
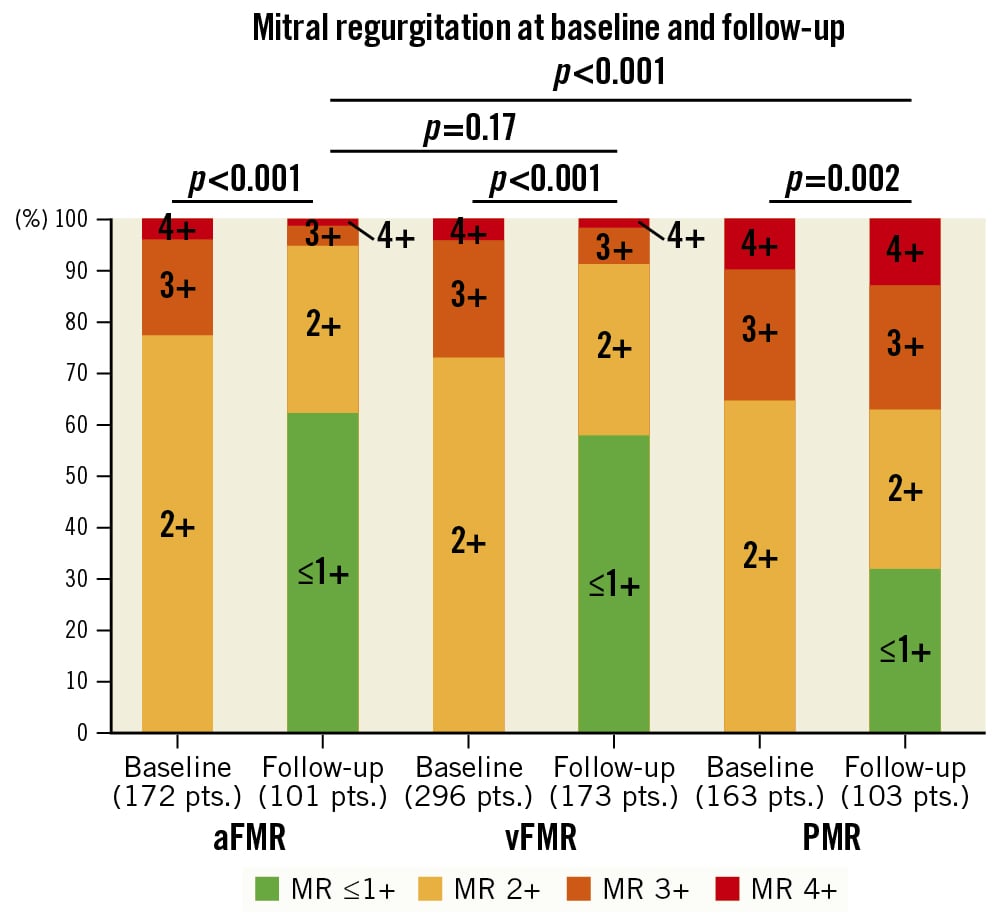
Figure 2. Mitral regurgitation at baseline and follow-up. This figure displays the changes in MR severity between baseline and follow-up for each MR aetiology. aFMR: atrial functional MR; MR: mitral regurgitation; PMR: primary MR; vFMR: ventricular functional MR
Table 3. Logistic regression model for MR persistence*.
| Characteristic | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Age, years | 1.01 | 0.99-1.01 | 0.32 | ||||
| BMI, kg/m² | 0.97 | 0.92-1.00 | 0.15 | ||||
| Sex (male) | 1.43 | 0.94-2.17 | 0.09 | ||||
| eGFR, ml/min | 0.99 | 0.98-1.00 | 0.59 | ||||
| STS score | 1.01 | 0.98-1.02 | 0.45 | ||||
| NYHA IV | 1.15 | 0.66-2.00 | 0.61 | ||||
| Hyperlipidaemia | 0.99 | 0.65-1.51 | 0.95 | ||||
| Hypertension | 1.07 | 0.59-1.99 | 0.83 | ||||
| Smoking | 0.96 | 0.55-1.66 | 0.89 | ||||
| Diabetes | 1.33 | 0.84-2.13 | 0.22 | ||||
| Positive family history | 1.49 | 0.73-3.00 | 0.27 | ||||
| COPD | 1.25 | 0.70-2.20 | 0.45 | ||||
| Coronary artery disease | 1.15 | 0.70-1.70 | 0.58 | ||||
| Previous MI | 1.30 | 0.80-2.20 | 0.34 | ||||
| Previous PCI | 0.95 | 0.60-1.47 | 0.80 | ||||
| Previous CABG | 1.26 | 0.61-2.50 | 0.51 | ||||
| Atrial fibrillation | 1.10 | 0.70-1.70 | 0.66 | ||||
| Prior biological AV prosthesis | 0.66 | 0.30-1.40 | 0.29 | ||||
| Prior cardiac surgery | 0.96 | 0.60-1.70 | 0.90 | ||||
| LVEF, % | 0.99 | 0.98-1.01 | 0.36 | ||||
| LVEDV index, ml/m2 | 1.00 | 1.00-1.01 | 0.68 | ||||
| LAV index, ml/m2 | 1.01 | 1.00-1.02 | 0.008 | 1.01 | 1.00-1.02 | 0.01 | |
| AV dpmean, mmHg | 0.99 | 0.97-1.00 | 0.06 | ||||
| AV opening area, cm² | 1.02 | 0.40-2.60 | 0.90 | ||||
| Mitral regurgitation grade ≥3+ | 1.05 | 0.65-1.68 | 0.86 | ||||
| Mitral annular diameter, mm | 1.02 | 0.99-1.05 | 0.30 | ||||
| Biplanar vena contracta, cm | 2.30 | 0.80-6.70 | 0.11 | ||||
| Tricuspid regurgitation grade ≥3+ | 1.20 | 0.60-2.20 | 0.58 | ||||
| RAV index, ml/m² | 1.00 | 0.90-1.01 | 0.47 | ||||
| RV mid-ventricular diameter, mm | 0.99 | 0.97-1.01 | 0.72 | ||||
| TAPSE, mm | 0.96 | 0.92-1.00 | 0.05 | 0.97 | 0.92-1.02 | 0.20 | |
| RV/RA gradient, mmHg | 1.00 | 0.99-1.01 | 0.80 | ||||
| MR aetiology | aFMR | 0.50 | 0.30-0.90 | 0.03 | 0.50 | 0.27-0.95 | 0.037 |
| vFMR | Reference | Reference | Reference | Reference | Reference | Reference | |
| PMR | 2.87 | 1.70-4.80 | <0.001 | 2.73 | 1.60-4.80 | <0.001 | |
| *MR persistence was defined as no change or worsening of MR severity at last available follow-up. aFMR: atrial functional mitral regurgitation; AV: aortic valve; BMI: body mass index; CABG: coronary artery bypass graft; CI: confidence interval; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; LAV: left atrial volume; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MR: mitral regurgitation; NYHA: New York Heart Association; OR: odds ratio; PCI: percutaneous coronary intervention; PMR: primary mitral regurgitation; RA: right atrium; RAV: right atrial volume; RV: right ventricle; TAPSE: tricuspid annular plane systolic excursion; vFMR: ventricular functional mitral regurgitation | |||||||
Three-year survival
After 3 years, the estimated overall survival rate was 55% (95% CI: 51-60) in patients with at least moderate MR. Estimated 3-year survival rates were similar between the three groups, with 58.2% (95% CI: 51-67) for aFMR patients, 53.2% (95% CI: 48-60) for vFMR patients and 56.4% (95% CI: 49-65) for PMR patients (p=0.58 by log-rank test) (Figure 3). Estimated 3-year survival rates were significantly lower among patients with MR ≥3+ (MR ≥3+ vs MR 2+: 48.5% vs 57.6%). Baseline MR ≥3+ was associated with a worse prognosis after TAVI (HR 1.34, 95% CI: 1.02-1.78; p=0.037) (Figure 4A). When analysing each entity separately, this effect was driven by the PMR group (HR 1.80, 95% CI: 1.08-3.00; p=0.023), while survival rates for MR ≥3+ and MR 2+ were similar in both functional MR groups (MR ≥3+ vs MR 2+ in aFMR: HR 1.20, 95% CI: 0.66-2.18; p=0.6; MR ≥3+ vs MR 2+ in vFMR: HR 1.17, 95% CI: 0.78-1.77; p=0.5) (Figure 4B, Figure 4C).
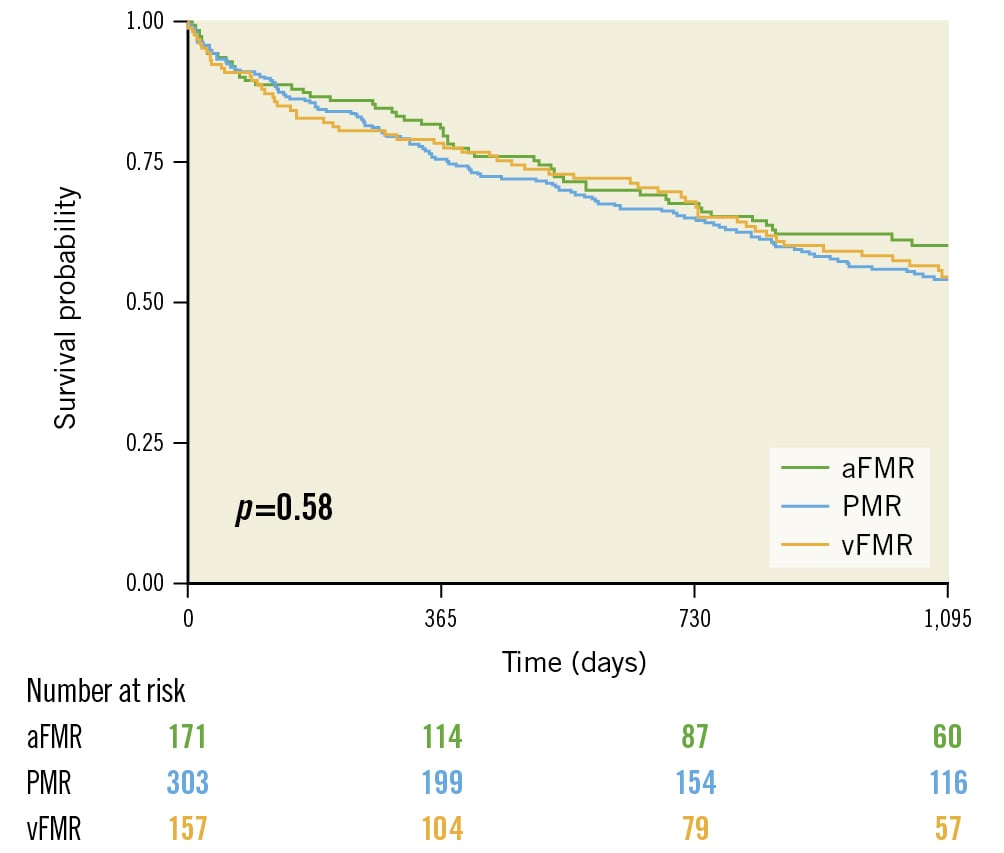
Figure 3. Survival according to MR aetiology. This Kaplan-Meier graph demonstrates the 3-year survival of all patients according to their MR aetiology (aFMR, vFMR and PMR). aFMR: atrial functional MR; MR: mitral regurgitation; PMR: primary MR; vFMR: ventricular functional MR
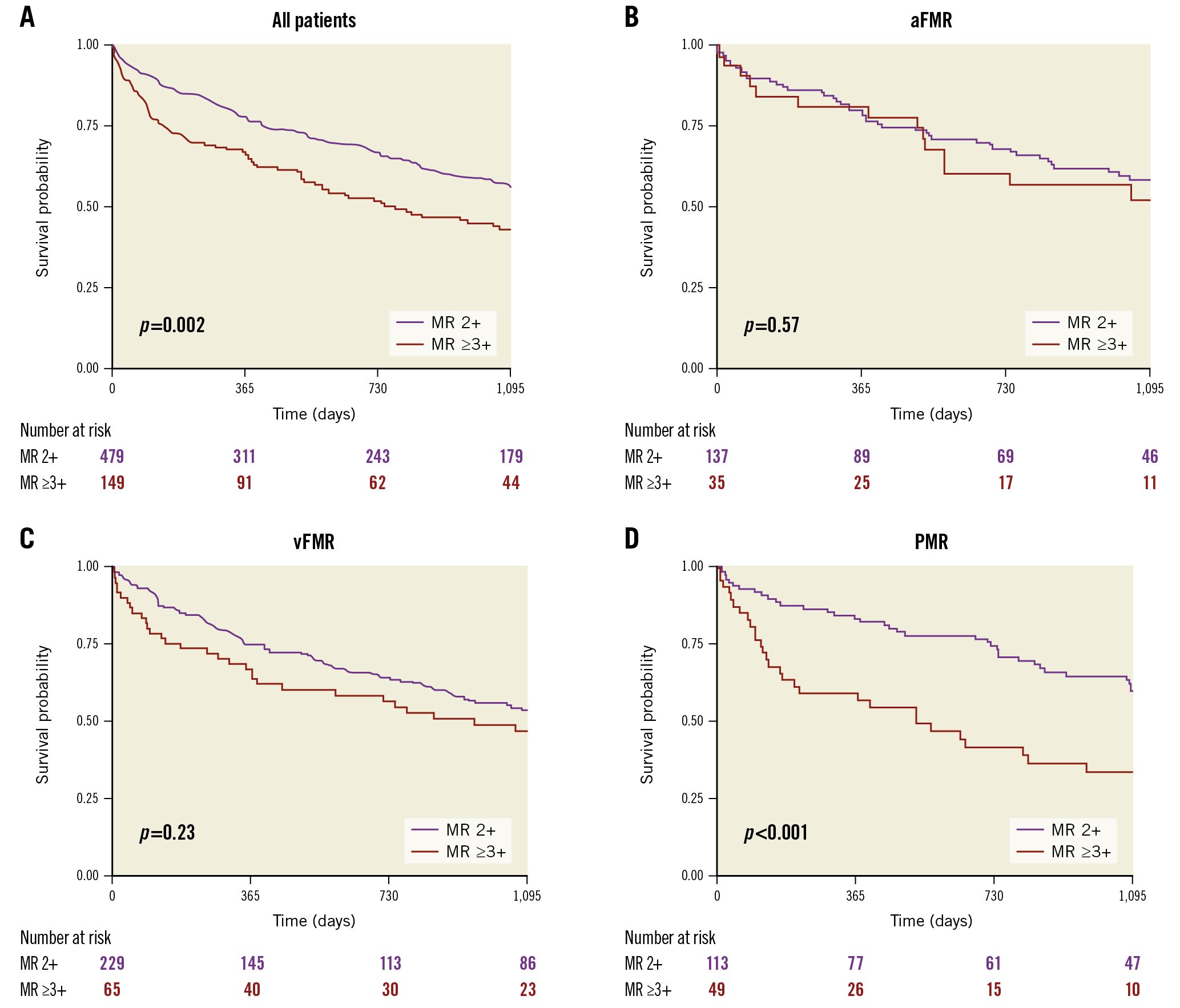
Figure 4. Survival of patients according to MR severity. A) The 3-year survival of all TAVI patients according to their baseline MR severity. B-D) Kaplan-Meier curves for each MR aetiology. aFMR: atrial functional MR; MR: mitral regurgitation; PMR: primary MR; TAVI: transcatheter aortic valve implantation; vFMR: ventricular functional MR
Symptomatic improvement
TAVI significantly reduced symptoms in all 3 groups (aFMR, vFMR and PMR; p<0.001 for comparison of baseline and follow-up in each group), with a more pronounced effect among vFMR patients (Supplementary Figure 3A). NYHA Class ≤II at follow-up was present in 82% of aFMR patients, 85% of vFMR and 84% of PMR patients. However, among patients with baseline MR ≥3+, the symptomatic improvement at follow-up differed significantly between groups. While 92.9% of aFMR and 86.2% of vFMR patients with MR≥3+ had an NYHA Class ≤II at follow-up, PMR patients with MR ≥3+ had the lowest symptomatic improvement following TAVI (NYHA ≤II: 69.6%; p=0.01 for PMR vs aFMR and p=0.03 for PMR vs vFMR, respectively) (Supplementary Figure 3B).
Discussion
This study describes the characteristics and outcomes of TAVI patients with significant concomitant MR of different aetiologies. We demonstrate that increased MR severity at baseline contributes to worse outcomes following TAVI. We further demonstrate that a considerable amount of TAVI-treated patients have aFMR and that TAVI can effectively reduce MR severity and symptoms in these patients. MR aetiology was shown to have a significant impact on MR severity improvement after TAVI, and MR persistence was associated with increased mortality.
The impact of MR, especially PMR, on the risk of death is controversial. Several studies have suggested an association between relevant MR and mortality, while other trials have not926. However, most larger trials, including our detailed analysis, have highlighted the prognostic relevance of significant concomitant MR among TAVI patients5689. To the best of our knowledge, this is the first analysis of TAVI patients with significant concomitant MR describing long-term outcomes with respect to different MR aetiologies, including the underrecognised entity of aFMR.
In some studies, the presence of PMR was associated with an increased mortality after TAVI, which might derive from a lower haemodynamic benefit in these patients due to a persistent structurally altered mitral valve. Vollenbroich et al suggested that the heterogeneity of the reported findings in the above-mentioned studies may result from the neglected differentiation of MR aetiologies6. Therefore, we aimed to address this gap in knowledge by analysing a large cohort of TAVI patients and performing a precise and comprehensive characterisation of MR aetiology, including the underrecognised entity of aFMR.
While TAVI was effective and associated with both symptomatic and MR severity improvement in all groups, these effects were significantly less pronounced in PMR patients. Additionally, more severe MR was not associated with significantly increased mortality among aFMR or vFMR patients, while PMR patients showed higher mortality rates when the baseline MR was ≥3+.
Haemodynamic aspects
MR severity assessment is challenging in patients with AS, because the regurgitant volume is aggravated by LV pressure overload. Importantly, TAVI positively impacts LV haemodynamics and has been shown to induce reverse remodelling27.
Thus, it seems intuitive that afterload reduction following TAVI can reduce MR severity. Conversely, despite the reduction in afterload, the structural alterations of the mitral valve in PMR patients negatively influence the potential for MR improvement and outcome in these patients. This thesis is supported by the observations that (i) PMR patients showed higher rates of MR persistence following TAVI and (ii) PMR remained a predictor for MR persistence after adjustment in multivariable logistic regression analysis. Therefore, a dual-valve intervention might be beneficial for these patients, as a sufficient MR reduction after TAVI is most probably not expected.
Subentity of atrial functional MR
Besides the impaired prognosis of PMR patients, we are able to demonstrate for the first time the outcome of patients with aFMR following TAVI. A precise characterisation of aFMR is challenging, therefore, in this study, patients were considered to have aFMR when showing preserved left ventricular ejection fraction, with normal indexed LV body surface area dimensions and without any regional wall motion abnormalities, a Carpentier type I leaflet motion, dilated left atria and an absence of leaflet calcifications. However, a standardised definition of aFMR is still lacking. Despite the fact that mitral annular dilation caused by left atrial enlargement is the leading underlying pathophysiology in aFMR patients, most recent literature does not include annular dilation in the proposed 4-pillar definition of aFMR22. This can be explained by the fact that AP diameter measurement, especially in the case of annular calcification, is highly prone to false measurements. This also explains the borderline dilation of the mitral annulus measured in aFMR patients of this study. aFMR typically occurs in the context of atrial fibrillation and/or HFpEF with severe LA dilation and LA pressure elevation which contributes significantly to the pathophysiology of FMR28. A reduction of afterload with consequent LV pressure reduction reduces the regurgitant volume in FMR patients, and especially in aFMR patients, due to the sustained left ventricular function. However, due to the lack of data for aFMR patients in general, the therapeutic management and especially the outcome after TAVI is unknown. This is the first study precisely characterising patients with the underrecognised subentity of aFMR in the context of TAVI.
Comparison of atrial and ventricular functional MR
We were able to demonstrate that aFMR patients show similar and, to some extent, even better procedural and long-term outcomes compared to vFMR patients. Additionally, we demonstrate that the presence of aFMR was associated with a lower risk of MR persistence after TAVI. Thus, the reduction of LV pressure seems to be beneficial for both aetiologic subgroups of functional MR, but the benefit appears to be even more pronounced in aFMR patients. Therefore, in patients with functional MR, a “watchful waiting” strategy, as suggested by current American and European guidelines2930, seems preferable, especially in aFMR patients, as MR severity and symptoms have a high potential for improvement. Moreover, despite their mutual functional aetiology, the distinction between vFMR and aFMR seems to be of certain importance regarding the rate of MR improvement. Potentially, in vFMR patients, the ventricular venting effect was less important, as these patients also suffer from mainly ischaemic cardiomyopathy with higher rates of LV function impairment, illustrated by the lower transvalvular gradients. These characteristics are known to be associated with worse outcomes after aortic valve replacement17.
Yet, the impact of untreated atrial fibrillation or progressive HFpEF on aFMR severity in the long run remains uncertain. Additionally, early antiarrhythmic treatment of atrial fibrillation might prevent the incidence or progression of aFMR. Future trials addressing these questions are necessary.
Limitations
Several limitations must be acknowledged that mostly derive from the retrospective nature of the study. Therefore, assumptions regarding the benefit of TAVI compared to optimal medical therapy cannot be made. Also, missing echocardiographic follow-up information in about 38% of patients, as well as missing additional indices for MR severity, have to be acknowledged. In addition, unknown but expected differences in heart failure medication should be acknowledged as potential confounders. Nevertheless, this remains the first study with a precise differentiation of aFMR, vFMR and PMR in a large cohort of TAVI-treated patients.
Conclusions
TAVI can effectively reduce MR severity and symptoms in patients with aFMR or vFMR, but the reduction is less pronounced in PMR patients. Therefore, for patients with severe AS and functional MR, a “watchful waiting” strategy might be preferable, as MR severity and symptoms have a high potential for improvement, especially in patients with aFMR. In contrast, the presence of PMR was associated with higher mortality, increased MR persistence and less symptomatic improvement following TAVI. In cases of severe AS and PMR, a dual-valve intervention might be an option in selected patients, since sufficient MR reduction after TAVI is most probably not expected.
Impact on daily practice
MR aetiology and severity should be considered when planning TAVI, and this detailed information may facilitate decision-making in this population. Further studies specifically evaluating HFpEF- and atrial fibrillation-associated aFMR, as well as the effect of atrial fibrillation treatment on aFMR, will significantly improve our understanding about this important subaetiology of FMR in the context of TAVI.
Conflict of interest statement
D. Braun reports speaker honoraria from Abbott Vascular and Edwards Lifesciences. J Hausleiter has received speaker honoraria from Abbott Vascular and Edwards Lifesciences. J. Steffen reports speaker honoraria from AstraZeneca. S. Deseive has received speaker honoraria from AstraZeneca. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

