
Abstract
Aims: The German healthcare system was among the first to introduce transcatheter mitral valve repair (TMVR) into routine care. The objective of this study was to analyse adoption and utilisation patterns and to estimate the impact of TMVR availability on mitral valve (MV) procedure volumes in the first eight years after commercialisation.
Methods and results: Procedure volumes were collected from German Federal Statistics Office databases for TMVR and mitral valve surgery (MVS) from 2008-2015. Procedure volumes were stratified by age group (<65, 65-74, 75-84, ≥85 years). Overall procedure volumes grew from 14,525 to 24,898 (+71%). MVS procedures grew from 14,477 to 20,402 (+41%) (p=0.008). The proportion of TMVR procedures grew from 0.3% (48 procedures) to 18.1% (4,496 procedures) (p=0.008). In 2015, TMVR use reached 5%, 15%, 31%, and 68% of overall MV procedures in the studied age groups (<65, 65-74, 75-84, ≥85 years). MVS volumes grew in all age groups, with the highest increase in the age group <65 (+2,945).
Conclusions: The availability of TMVR has contributed to a pronounced increase in MV procedure volumes in Germany. Simultaneously, MVS procedure volumes continued to grow substantially. The highest increase of TMVR was observed in elderly populations, suggesting referral of patients with MV disease previously left untreated.
Abbreviations
COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy
DRG: diagnosis-related group
EAC: early adopting centre
EVEREST: Endovascular Valve Edge-to-Edge Repair Study
GSTCVS: German Society for Thoracic and Cardiovascular Surgery
ICD-10-GM: International Statistical Classification of Diseases and Related Health Problems, revision 10, German modification
ICPM: International Classification of Procedures in Medicine
LV: left ventricle/ventricular
MATTERHORN: Multicenter, Randomized, Controlled Study to Assess Mitral vAlve reconsTrucTion for advancEd Insufficiency of Functional or iscHemic ORigiN
MC: MitraClip
MR: mitral regurgitation
MVS: mitral valve surgery
NUB: Neue Untersuchung- und Behandlungsmethoden (new examination and treatment methods)
OPS: Operationen- und Prozedurenschlüssel (operation and procedure code)
TMVR: transcatheter mitral valve repair
TRAMI: transcatheter mitral valve interventions registry
Introduction
According to guideline-directed treatment schemes, severe symptomatic mitral regurgitation (MR) or asymptomatic chronic severe primary MR with left ventricular (LV) dysfunction or enlargement indicates mitral valve surgery (MVS) utilising repair or replacement. Also, concomitant MVS in patients with severe chronic primary MR or chronic moderate-severe secondary MR who undergo cardiac surgery for different indications is justified1. However, only 50% of patients suffering from relevant MR receive surgical treatment due to impaired LV ejection fraction, advanced age and/or relevant comorbid conditions with associated high surgical risk2. The clinical availability of transcatheter mitral valve repair (TMVR) has introduced new therapeutic options for high-risk patients formerly ineligible for causal treatment. Among the commercially available TMVR systems, most experience has been gathered with the MitraClip® (MC) device (Abbott Vascular, Santa Clara, CA, USA)3,4.
The MC system was initially evaluated in the EVEREST I (Endovascular Valve Edge-to-Edge Repair Study) and EVEREST II trials, resulting in FDA approval for high-risk patients with degenerative MR ineligible for MVS5-7. Furthermore, the efficacy of the MC device is currently being evaluated against best medical therapy in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy) randomised controlled trial8 and against MVS in the MATTERHORN (Multicenter, Randomized, Controlled Study to Assess Mitral vAlve reconsTrucTion for advancEd Insufficiency of Functional or iscHemic ORigiN) trial9. In Europe, TMVR using the MC system is predominantly used as an adjunctive heart failure therapy in inoperable patients with functional MR but is also approved for primary MR. In the USA, MC is only approved for primary MR in patients with severe symptoms who are at prohibitive risk for surgery10.
A previous study reported on the first years of experience with TMVR and concurrent development of surgical volumes at an early adopting centre (EAC) in Germany during the period 2008-201211. The main finding of that study was that, concurrent to adoption of TMVR, MVS procedure volumes continued to exhibit meaningful growth.
The primary objective of the present study was to update these prior findings by extending the study time period to 2015, and to provide a more detailed evaluation of adoption patterns and growth trends in various age groups at the German national level. This extended analysis is of particular relevance as TMVR indications will probably continue to expand12, and Heart Teams evaluate their decision processes based on experience gained in earlier years. Furthermore, analysis of the more recent national data provides insight into TMVR adoption patterns observed with more widespread adoption of the therapy and additional initiation of TMVR programmes across the country. These data are of relevance beyond the German context, as similar patterns may emerge across other healthcare systems in Europe and North America.
Methods
DATA COLLECTION
MVS and TMVR procedure volumes were assessed for the period 2008 to 2015 at the German national level. Respective procedural volume data were collected for relevant mitral valve procedure codes and were collected from national records as previously described11: relevant procedure codes (referred to as Operationen- und Prozedurencodes [OPS]; OPS codes represent the German national modification of the “International Classification of Procedures in Medicine” [ICPM]) were prospectively identified using the following approach (Supplementary Table 1 for OPS codes utilised):
– search annual OPS catalogues from 2008 until 2015 for codes containing the word “mitral”
– exclude all OPS codes containing the word “congenital”
– exclude OPS codes not relevant in the context of the current study (among them change of bioprosthetic or mechanical valves, treatment of mitral stenosis, thrombectomy)
Therapy- and age-stratified procedure volumes were collected from the German Federal Statistical Office for the years 2008-2015. Selected age groups were defined as follows: <65, 65-74, 75-84, and ≥85 years.
To evaluate changes in reimbursement, diagnosis-related group (DRG) reimbursement amounts for the respective codes and years were collected from published records, using respective DRG base rates of the applicable federal state (Supplementary Appendix 1).
MORTALITY RATES
Nationwide data regarding mortality are not documented at the German Federal Statistical Office. Therefore, in-hospital and 30-day mortality for the nationwide patient collective was implicitly acquired from already published data from the transcatheter mitral valve interventions (TRAMI) registry for MC therapy for the years 2010-2013 and the German Society for Thoracic and Cardiovascular Surgery (GSTCVS) for MVS for the years 2008-2015.
STATISTICAL ANALYSIS
Volumes were analysed annually for TMVR and MVS and stratified by age group. Growth rates were calculated on an annual basis, and for the specified analysis timeframe. All data are presented as absolute numbers and percentages for categorical variables. For statistical analyses of trends and differences in procedure volume growth rates – overall and by age group – a non-parametric test for trend across ordered groups (Cuzick’s test, an extension of the Wilcoxon rank-sum test, with correction for ties) was utilised. Testing was performed for the TMVR group, the MVS group, and for the difference between these two groups. For all analyses, statistical significance was determined based on a p-level of 0.05. All statistical analyses were performed using Stata 14 (StataCorp, College Station, TX, USA), and Excel 2013 (Microsoft Corporation, Redmond, WA, USA) software packages.
Results
NATIONAL PROCEDURE VOLUMES
In the study period, overall procedure volumes grew from 14,525 to 24,898 (+71%). MVS procedure volumes grew from 14,477 to 20,402 (+41%). TMVR procedures grew from 48 procedures in 2008 to 4,496 procedures in 2015. P for trend values were 0.008 for TMVR and for MVS, and 0.345 for the difference between the group growth rates, indicating statistically significant volume growth over time for both the TMVR and the MVS groups, but not for the difference between these two growth rates (Figure 1).
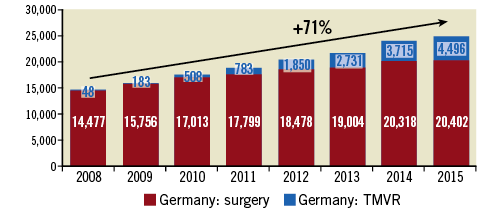
Figure 1. Total number of TMVR and surgical interventions at German national level, years 2008-2015.
RELATIVE PROPORTION OF TMVR PROCEDURES
The proportion of TMVR procedures as part of the overall MV procedures grew from 0.3% (48 procedures) in 2008 to 18.1% (4,496 procedures) in 2015. TMVR proportions continued to exhibit year-on-year increase across the study horizon. Statistical testing found a p for trend value of 0.008 for Germany (Figure 2).
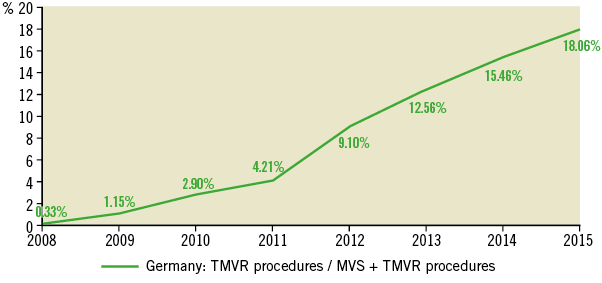
Figure 2. Percent of overall mitral valve procedures performed as TMVR at German national level, years 2008-2015.
PROCEDURE VOLUMES AND PROPORTIONS OF TMVR STRATIFIED BY AGE COHORT
In 2015, TMVR use reached 5%, 15%, 31%, and 68% of overall MV procedures in the studied age groups (<65, 65-74, 75-84, ≥85 years) (Figure 3). Compared to base year 2008, MVS volumes grew in all age groups over the eight-year study period, with the highest increase in the age group <65 (+2,945; +51.5%) and smallest increase in the age group ≥85 years (+85 procedures; +40.9%) (Supplementary Figure 1).
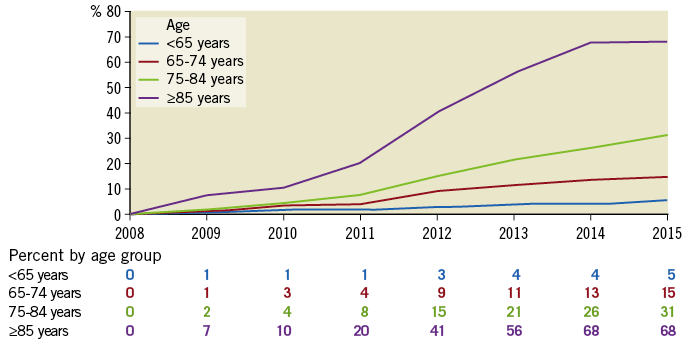
Figure 3. Percent of total mitral valve procedures performed as TMVR, by age group in Germany, years 2008-2015.
Absolute procedural volumes exhibited a statistically significant growth trend over time for TMVR across all age groups in Germany (p for trend= <65: 0.010; 65-74: 0.008; 75-84: 0.008; ≥85: 0.008). For MVS procedure volumes, positive p for trend values were observed in all age groups except for the age group ≥85 years (p= <65: 0.008; 65-74: 0.012; 75-84: 0.008; ≥85: 0.231) (Supplementary Figure 2).
For growth in the relative proportion of TMVR of overall procedure volumes, a positive p for trend was observed for all age groups in Germany (p for trend= <65: 0.010; 65-74: 0.008; 75-84: 0.008; ≥85: 0.008).
EVOLUTION OF REIMBURSEMENT
The per-case reimbursement for TMVR increased from 28,400 euros in 2008 to 35,500 euros in 2015 (+25%), attributable to gradual adjustments in the DRG reimbursement amounts for TMVR. Supplementary Figure 3 shows an example of reimbursement increase at a single centre.
IN-HOSPITAL AND 30-DAY MORTALITY
Isolated MVS in Germany presented an in-hospital mortality as documented annually by the GSTCVS: 4.9% in 2008, 4.0% in 2009, 4.0% in 2010, 4.5% in 2011, 4.1% in 2012, 4.1% in 2013, 4.1% in 2014 and 4.2% in 201513-20. Available data from the TRAMI registry from 2010 to 2013 presented an in-hospital and 30-day mortality for TMVR of 2.4% and 4.5%, respectively21.
Discussion
The findings of this study provide new insight into diffusion and adoption patterns observed for TMVR in the German healthcare system, which was the first to adopt TMVR in 2008. The results suggest that TMVR has seen significant adoption over the last few years with the proportion of TMVR procedures approaching one fifth of overall mitral valve procedure volumes. The increasing proportion of TMVR in relation to all MV procedures is probably due to additional referral of patients with MV disease previously left untreated because of severely impaired LV ejection fraction, advanced age and/or relevant comorbid conditions with associated high surgical risk. Since the introduction of TMVR into clinical routine, these patients with functional or degenerative MR can now be provided with an adequate therapy. Additionally, awareness of novel TMVR therapies has increased over recent years, resulting in allocation of a high-risk patient population from cardiologists.
In parallel to TMVR, MVS procedure volumes have continued to grow substantially, further corroborating findings from an earlier study on TMVR adoption experience suggesting a “halo effect” of TMVR on MVS. This growth can be partially attributed to established mitral valve centres providing experienced Heart Teams who evaluate allocated patients in an unbiased fashion. Thereby, patients who were initially assigned for TMVR are regularly transferred to MVS and vice versa. This effect is confirmed by the herein described robust growth rates of MVS at the EAC. On the other hand, this growth may also be driven by effects such as the increase of prevalence in an ageing population, improved diagnostic tools and a more liberal indication and referral policy22.
Previously unreported adoption patterns by age group suggest that TMVR has experienced the most pronounced growth in elderly patients, which underlines the genuine patient population for which TMVR was approved in Europe, i.e., elderly patients with functional MR and an increased risk for MVS. This particular subgroup of patients was also largely dominant in a recently published TMVR registry23. Furthermore, TMVR is rarely utilised in younger patients and only when prohibitive surgical risk is present or for other rare indications such as for bridge to transplantation24.
Evaluating procedure volume evolution at our centre in detail, it was observed that TMVR volumes, after years of high growth rates, have exhibited slower growth rates in more recent years, more closely resembling the increase observed in MVS volumes. This effect is possibly explained by increasing availability of sites offering TMVR across the country, which reduces the potential number of referrals to the EAC. A second hypothesis is that patients who are candidates for TMVR are effectively identified by the Heart Team approach, and the relative proportion of TMVR remains stable at an experienced MV centre. Supplementary Figure 4-Supplementary Figure 6 show comparisons of nationwide and single-centre growth rates of TMVR and MVS.
At the national level, a significant increase in TMVR procedures in all age groups was documented by the analysis. Significance testing for trends showed statistically significant growth trends for both TMVR and MVS. In our centre, we saw a non-significant TMVR growth trend for the age group ≥85 years over time which is largely due to an early steep uptake of TMVR procedures, and a more level volume development thereafter. Also, the diffusion of TMVR to surrounding centres may have contributed to this non-significance.
MVS procedure volumes continued to grow in all studied age groups after the introduction of TMVR until 2012. From then on, the age group of patients ≥85 years experienced a reduction in MVS volumes in Germany since TMVR experienced further growth in this population. At the same time, MVS procedure volumes saw the highest growth rates in patients <65 years of age, emphasising again the halo and crossover effect of a TMVR programme for MVS and overall mitral valve procedures.
Reimbursement analysis at our centre suggests continued increase in reimbursement revenues. While this increase is heavily driven by the adoption of TMVR, it is noteworthy that MVS procedure reimbursement concurrently more than doubled in the study period.
Limitations
The current analysis is subject to several limitations. First, it relies on volumes determined from procedure counts, as opposed to case counts. Changes in procedure coding and coding practices might have occurred over the study period and might also vary somewhat between individual hospitals. However, reliance on procedure codes provided a level of granularity in characterising the TMVR and MVS groups that would not have been possible by analysing DRG case volumes published by the national statistics office. Further, trends observed in procedure volumes can be expected also to reflect corresponding relative changes in overall case counts. Second, while our analysis considers volume development in different age groups, it does not consider other cohort characteristics and risk factors that would be of interest to study, and that might correlate with some of the observed volume changes. Third, volume developments in individual centres might be driven by a wide variety of factors. Finally, nationwide mortality rates were achieved implicitly and do not cover the whole timeframe investigated here. Therefore, there is a methodological inconsistency regarding comparison of TMVR and MVS mortality rates. Also, this work does not provide information about the clinical benefit of TMVR, the ideal patient population or optimal intervention timing for catheter-based mitral valve therapy.
Conclusions
In summary, our analysis suggests that the availability of TMVR has contributed to pronounced growth in overall MV procedure volumes in Germany. At the same time, MVS procedure volumes continued to grow substantially. The highest growth of TMVR was observed in elderly populations, suggesting referral of patients with MV disease previously left untreated. Halo and crossover effects contributed to a parallel increase in MVS procedures. This growth can be partially assigned to established mitral valve centres providing experienced Heart Teams who evaluate allocated patients in an unbiased fashion. Thereby, patients who were initially assigned for TMVR are regularly transferred to MVS and vice versa. A shift of intermediate-risk patients to a TMVR regimen, as observed in other transcatheter therapies for valvular heart disease25,26, seems unlikely; therefore, TMVR is an additional and not a competitive therapy for MVS.
| Impact on daily practice The introduction of TMVR into daily clinical routine led to a referral of patients with MV disease previously left untreated. Evaluation of these allocated patients in an unbiased fashion is of crucial importance to provide the best care for every patient. Experienced Heart Teams should be a mandatory prerequisite for this assessment. |
Funding
Wing Tech Inc. (J.B. Pietzsch, S. Weber, M. Pietzsch) provided health-economic consulting services to Abbott Vascular in support of this analysis and received compensation for its services. The authors maintained the right to publish without the approval of the funding source.
Acknowledgements
The authors thank Dr Benjamin Geisler (Wing Tech Inc.) for providing statistical analysis support.
Conflict of interest statement
A. Schaefer reports other from Abbott Vascular, outside the submitted work (travel support). J.B. Pietzsch reports other from Abbott Vascular, during the conduct of the study (compensation for health-economic consulting services). M. Pietzsch reports other from Abbott Vascular, during the conduct of the study (compensation for health-economic consulting services). S. Weber reports other from Abbott Vascular, during the conduct of the study (compensation for health-economic consulting services). L. Conradi reports other from Abbott Vascular, outside the submitted work (speaker honoraria, travel compensation). The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Computation of DRG reimbursement amounts.
Supplementary Table 1. List of included OPS procedure codes that were included in the analysis to determine volumes in each specific year.
Supplementary Figure 1. Procedure volumes by age group.
Supplementary Figure 2. Representation of MVS and TMVR procedure volumes at German national level, by age group.
Supplementary Figure 3. Estimated annual reimbursement amount of all TMVR cases, MVS, and resulting overall mitral valve reimbursement, University Heart Center Hamburg, 2008-2015.
Supplementary Figure 4. Comparison of nationwide and single-centre growth rates of TMVR and MVS from 2008 to 2015.
Supplementary Figure 5. Percentages of TMVR procedures as part of overall mitral valve procedures in Germany and at an early adopting centre.
Supplementary Figure 6. Percentages of TMVR procedures as part of overall mitral valve procedures in Germany and at an early adopting centre stratified by age group.
To read the full content of this article, please download the PDF.

