![]()
Abstract
France, with its 66 million inhabitants, has a long tradition in interventional cardiology, with numerous innovations and contributions to the dynamism of European activity. The development of interventional cardiology requires supervised training, organisation and participation in studies. This also translates into an ever-expanding clinical activity since 2010, such as the coronary and structural interventions that are detailed in the present review.
Introduction
From its earliest beginnings, interventional cardiology has been firmly anchored in the history of France. Of the many pioneers in the field, three names particularly come to mind: Jacques Puel was the first to implant a coronary stent in 19861, Paul Barragan contributed extensively to the development of stenting by proposing the introduction of post-procedural dual antiplatelet therapy2, and finally Alain Cribier was at the forefront of one of the recent major advances in cardiology, the TAVI procedure3.
Demographics and organisation
France is a relatively large country in Europe (550,000 km2) which today counts 204 interventional cardiology centres (114 public centres, including 34 university-affiliated, and 90 private centres), or approximately 1 per 320,000 inhabitants. Fifty-nine of the 204 teams (29%) have cardiac surgery on site. The number of French cardiologists engaged in regular interventional activity is estimated at 1,000. The Coronary Atheroma and Interventional Cardiology Group or GACI (Groupe Athérome coronaire et Cardiologie Interventionnelle) is the group of the French Society of Cardiology (SFC) which coordinates the activity of interventional cardiology. It is headed by a Board consisting of eight elected members (four public sector and four private sector cardiologists), renewed every two years. The GACI website (www.sfcardio.fr/groupe-atherome-coronaire-et-cardiologie-inter
ventionnelle) relays information on interventional cardiology with downloadable agendas, presentations and recommendations, as well as progress updates, and provides links to other sites.
The recommendations published in 2000 by the French Society of Cardiology with regard to training of physicians, organisation, equipment for coronary angiography and coronary angioplasty centres are still the mainstay4.
In accordance with the decree on interventional cardiology published in 2009, a centre must be able to manage coronary emergencies 24 hours a day, 365 days a year and have a cardiology intensive care unit. A minimum of 350 percutaneous coronary interventions (PCI)/year is required for each centre. Each centre must have at least two experienced operators.
A close collaboration with a cardiac surgery centre is necessary for discussion of complex Heart Team cases or for the implantation of circulatory support systems or for urgent surgical indications.
A minimum of 600 angioplasties per year is required for the centre to be considered as a training centre.
Interventional cardiology training is ensured by both theoretical and practical training (500 coronary angiographies and 300 angioplasties of which half as first operator) over two years as part of a national diploma requirement for interventional cardiology practice and delivered by GACI and the French Society of Cardiology.
Coronary interventions
The number of coronary angioplasties continues to rise in France despite the marked reduction in activity related to the treatment of restenosis (<4%). The ageing of the population, the systematic exploration of acute coronary syndromes and expansion of indications supported by technological advances largely explain this constant progression.
Table 1 summarises the figures reported annually by Didier Blanchard based on data from the industry.

The number of coronary angiographies has experienced a slow but steady growth with the number of exams reaching 280,000 in 2015. The number of angioplasties has also increased to 128,000, one fifth of which were performed during acute STEMI. In 2013, there were eight angioplasty procedures per one bypass surgery (a total of 117,000 PCI vs. 14,500 CABG in France).
In two thirds of cases, angioplasty is performed in the same procedural time as coronary angiography. The use of the radial access and drug-eluting stents (DES) has systematically increased, reaching 80% and 83%, respectively, in 2015. These latter procedures are widely favoured including in primary angioplasty.
Bare metal stents (BMS) or DES are covered by health insurance as are the procedures and hospitalisations, subject to compliance with recommendations. Conversely, bioresorbable vascular scaffolds (BRS) are not currently reimbursed. The latter represent only 1.3% of stents implanted in France, although this number is on the rise, particularly in the population of young patients, as demonstrated in the France-ABSORB registry currently under analysis. There is also a steady increase in the number of rotational atherectomies.
Fractional flow reserve (FFR), which has only been reimbursed since 2016, should accelerate its progression (15,000 FFR in 2015, double the activity recorded in 2010 and nearly 12% of all angioplasties).
Recourse to intravascular imaging (intravascular ultrasound [IVUS] and optical coherence tomography [OCT]) is conversely more subtle (a total of 4,500 procedures in 2015) and remains non-reimbursed. Since 2013, OCT is used at a greater frequency than IVUS and is available in nearly 100 French centres.
The management of acute coronary syndrome (ACS) represents a substantial activity of interventional cardiology centres. It is the justification of the large number of centres necessary for ensuring territorial coverage. Primary angioplasty is the established treatment of STEMI, with the vast majority of the population having the possibility for revascularisation in less than 90 min. As recommended, coronary angiography is also performed increasingly early in the treatment of NSTEMI5-7.
France can rely on an effective collaboration between emergency physicians, the emergency medical assistance system (SAMU) and interventional cardiologists, although time to management still has to be optimised. Indeed, the emergency alert delay is decreasing but only half of patients directly call SAMU, with a real waste of time for those who contact their personal doctor. This ultimately involves better dissemination of information to the general public, including promoting a message encouraging the population to dial a single number (no. 15) in the event of chest pain, so as to limit the number of intermediaries and shorten the delay times.
Important data have been provided by registries conducted by Danchin et al (USIK trial8,9, USIC [Unités de Soins Intensifs Coronaires] survey10, and FAST-MI Registry [French Registry on acute ST- and non-ST-segment elevation myocardial infarction]5,11) (Table 2, Figure 1). The last of these represents one-month snapshots taken every five years, compiling all managed ACS cases over the entire territory. The strength of this registry resides in the comprehensiveness and quality of the collected data.
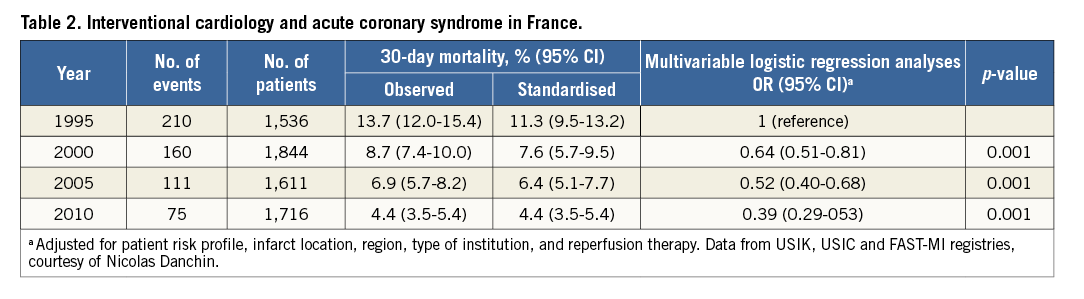
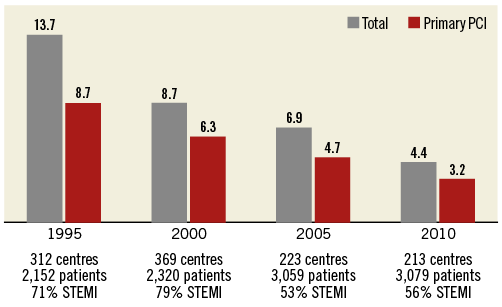
Figure 1. One-month mortality in STEMI in France. (Data from USIK, USIC and FAST-MI registries, courtesy of Nicolas Danchin)
Epidemiological as well as management data pertaining to prognosis have thus been analysed, enabling a longitudinal follow-up of practices and their impact5. This analysis revealed that the 30-day mortality of patients treated for STEMI dropped threefold between 1995 and 2010 (13.7 to 4.4%) and continues to decrease. This improved prognosis can be explained by a shorter time delay from onset to first call, decreasing from 120 to 74 min (median values), an initial pathway based on mobile ICU (from 23.2 to 48.8%) and the rate of patients accessing primary angioplasty (from 19.5% to 86.7%). Fibrinolysis, while still practised, notably in rural areas, represented less than 14% of STEMI in 2010. Mortality was the lowest for primary angioplasty, although prognosis was also being improved in the absence of reperfusion, thus demonstrating the progress achieved in management as a whole, and particularly in medical treatment. As a result, the use of evidence-based treatments during the first 48 hours from admission has gradually increased over the span of 15 years (β-blockers +15.5%; ACE inhibitors or angiotensin-receptor blockers +17.1%; statins +80.1%; antiplatelet agents +5%).
Data from FAST-MI 2015, which includes close to 5,300 ACS patients admitted in 203 centres, are in the process of being analysed and should be reported shortly. These data will allow determining whether the advances observed in 2010, largely due to adherence to the European recommendations, are confirmed and translate into better survival.
TRANSCATHETER AORTIC VALVE IMPLANTATION (TAVI)
France remains at the forefront in the field, with an activity in full expansion and for which the follow-up is assured by a national registry, France TAVI.
This registry was built with an electronic database supported by the French Society of Cardiology and thanks to partial funding by Edwards and Medtronic. Data were prospectively collected by each interventional centre and centralised for independent analysis after monitoring by a supervisory committee and dedicated research team of the French Society of Cardiology.
The currently recognised indications are severe symptomatic aortic stenoses in patients unsuitable for surgery or considered at high surgical risk12. Only two types of prosthesis are currently reimbursed (Edwards SAPIEN [Edwards Lifesciences, Irvine, CA, USA] and Medtronic CoreValve® [Medtronic, Dublin, Ireland]). Over 15,000 prostheses have been implanted percutaneously over a span of three years (2013-2015) in 50 centres with heart surgery capabilities, the latter being the only centres authorised to perform TAVI in France.
In 2015, 6,919 TAVI procedures were performed throughout the country (i.e., 138 TAVI/centre on average). The average age of treated patients was 83.4 years with a EuroSCORE of 17.4.
Femoral access is the preferred choice (85%), with the procedures most often performed without general anaesthesia (56%) with shorter hospital stays (7 days). Operator experience and advances in device technology have reduced the rate of complications, including bleeding. The main complication remains conduction disturbances requiring implantation of a pacemaker once every six TAVI procedures. Procedural mortality was less than 1% in 2015, with hospital mortality accounting for 3%.
In the light of the most recent studies, current indications are expected to expand to intermediate-risk patients with lower mean age and risk score.
Structural heart interventions (Table 3)
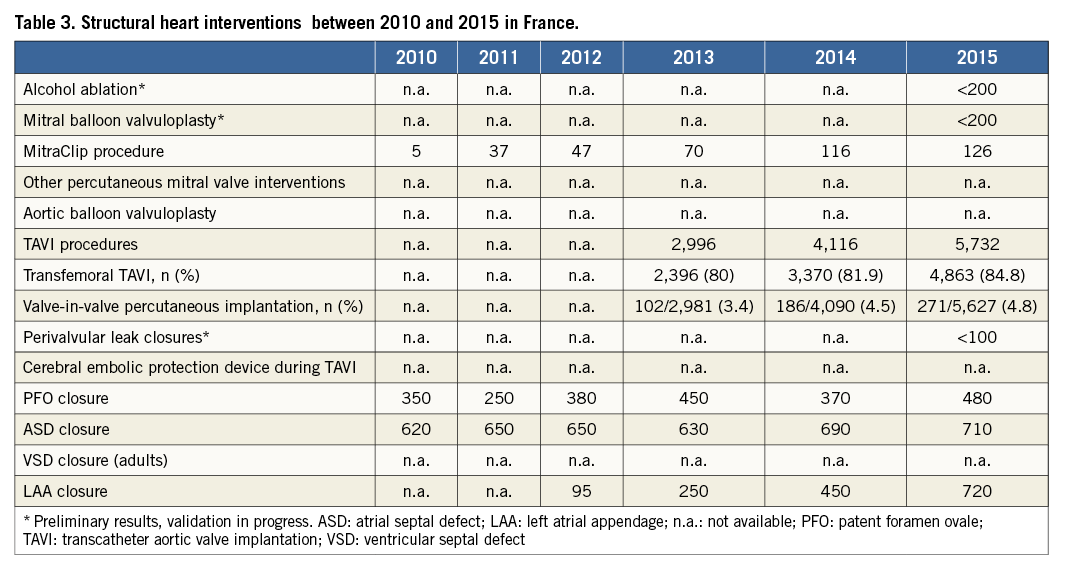
ASD and PFO closures are covered by health insurance in the setting of recognised indications. The volume of activity remains stable in France, with procedures being performed in only a few centres. An expert consensus in 2005 has validated PFO closure in the treatment of platypnoea-orthodeoxia syndrome. The management of decompression sickness and paradoxical embolism in cryptogenic stroke is however more contentious. With regard to ASD closure, in accordance with European recommendations, device closure has become the first choice for secundum atrial septal defect closure when feasible from morphology.
The disappearance of rheumatic mitral stenosis explains the lack of mitral valvuloplasties performed in France, with an estimated number of less than 200 procedures/year.
Alcohol septal ablations for obstructive hypertrophic cardiomyopathy are performed in 15 centres in France with a stable number of about 200 procedures/year.
Paravalvular leak closures, performed in less than 10 centres, are rare, albeit increasing.
PERCUTANEOUS REPAIR OF MITRAL REGURGITATION (MITRACLIP)
Mitral regurgitation is the second most frequent valve disease in Europe. The French National Authority for Health (HAS, Haute Autorité de Santé), in accordance with European guidelines, has validated the indications of MitraClip® (Abbott Vascular, Santa Clara, CA, USA) in patients with severe mitral regurgitation: these include a degenerative origin, symptomatic despite optimal medical management, contraindication for surgery (Heart Team decision), with a life expectancy >1 year and meeting echocardiographic criteria for eligibility.
The necessary prerequisites for the implantation of the MitraClip are a medico-surgical centre featuring a cathlab and cardiac surgery at the same site, as well as the collaboration of a team comprising an interventional cardiologist, a cardiac surgeon, a cardiologist specialised in echocardiography and an anaesthetist.
The number of procedures is steadily increasing in France, with 236 implantations between 2010 and 2014 and 126 implantations for the year 2015.
Most of the procedures are performed as part of the MITRA-FR study (NCT01920698), which is a multicentre, randomised study aimed at comparing the MitraClip to optimal medical treatment in patients with severe secondary mitral regurgitation (primary outcome measure combining death and hospitalisation for heart failure). Twenty French centres are currently participating with the inclusion of 288 patients expected for 2017. In the future, the training of teams and reimbursement of the procedure, obtained in 2016, should accelerate its diffusion.
PERCUTANEOUS CLOSURE OF THE LEFT ATRIAL APPENDAGE
The indication is validated for patients with non-valvular atrial fibrillation (AF) at high thromboembolic risk (CHA2DS2-VASc score ≥4) with a formal and permanent contraindication to anticoagulants. Two devices are available in France and reimbursed since mid 2016 (WATCHMAN® [Boston Scientific, Marlborough, MA, USA], and AMPLATZER™ Cardiac Plug [St. Jude Medical, St. Paul, MN, USA]).
The French recommendations establishing the indications (multidisciplinary decision) and conditions for implantation have been published by a committee of experts13. Implantation is currently limited to centres with cardiac surgery facilities, with a threshold of activity of 25 implantations per year, to be reached within three years.
The FLAAC (French Database of Left Atrial Appendage Closure) registry (PI: Emmanuel Teiger, NCT02252861) was established in 2013 allowing the compilation of all procedures and features in 41 participating centres with the projected goal of following 800 patients by 2020. The preliminary results of the first 93 patients are encouraging with a 100% implantation success rate and rare periprocedural complications at one year (one tamponade, one prosthesis embolisation at one month, and one massive haemorrhagic stroke at six months).
Left atrial appendage closure should witness a strong growth in the coming years in France, given the size of the eligible population. The reimbursement of the procedure obtained in 2016 should allow making up part of the observed delay relative to other European countries.
Ongoing projects
Among its various missions, the GACI has the role of encouraging and supporting registry projects.
France-ABSORB has enabled including 2,000 consecutive implantation procedures of the Absorb BVS (Abbott Vascular) between October 2014 and April 2016 (87 centres). The data are currently under analysis (PI: René Koning).
France TAVI provides the most extensive follow-up of implanted TAVI in France. More than 15,000 TAVI procedures performed over the past three years in 50 centres are being followed (PI: Hervé Le Breton).
Mitragister is a French registry of percutaneous treatment of the mitral valve (PI: Patrice Guerin).
In the study of the Prevalence Fibromuscular Dysplasia in Patient With Haematoma or Spontaneous Coronary Artery Dissection (DISCO), prevalence is being investigated by genetic testing and non-invasive imaging of the arteries in a patient population (200 patients expected in two years) having presented a spontaneous haematoma or coronary artery dissection. A total of 40 French centres are currently participating in the study (PI: Pascal Motreff, NCT02799186).
INFOCORO is a project led by young GACI-SFC fellows, aimed at evaluating, in a randomised study and using a dedicated questionnaire, the incremental value of a short educational video about coronary angiography compared to conventional information, in terms of patient’s understanding, satisfaction and anxiety (PI: Benoit Lattuca, NCT02484144).
Other registries such as ANOCOR RISK (registry of at-risk congenital coronary abnormalities, PI: Pierre Aubry), WAMIF (registry of myocardial infarction in young women, PI: Stephane Manzo-Silberman), ENCOCHE (registry of CTO procedures, PI: Nicolas Boudou), SOS PK (registry of PK Papyrus [Biotronik, Berlin, Germany] covered stent implantations, PI: Loic Belle) will soon begin.
AMBITION OF THE GACI: FRANCE-PCI
While there are regional registers, for example the FAST-MI project pertaining to ACS, there is no national registry such as that developed in Sweden (SCAAR).
The ambition of the GACI is to build a nationwide registry as quickly as possible to become a sustainable source of data: France-PCI. From this registry, we will be able to specify, for example, the number of angioplasties of the common core, CTO procedures, the specifics regarding primary angioplasties (delays, thromboaspiration rates, two-stage strategies, revascularisation of non-culprit lesions), thrombosis and stent restenosis rates, lengths of stay, the safety of outpatient procedures. Such a registry will not only provide general data but also the opportunity for each centre to compare itself with the national average, and to improve predefined quality indicators. Four of the 12 French regions should adhere in 2017 allowing coverage of 17 million inhabitants, more than a quarter of the population. There is a national consensus among cardiologists to support this project. Discussions are underway to receive support and funding for France-PCI from the health authorities.
Conclusion
Interventional cardiology in France is dynamic with an adequate provision of healthcare covering the entire French territory. While the treatment of coronary heart disease represents the largest activity of French cathlabs, procedures including TAVI and structural treatment occupy an increasingly significant place. The challenge ahead is to provide, to as many as possible, access to therapies, albeit expensive but effective, and to maintain quality of care in a difficult economic context. Development of interventional cardiology will require systematic evaluation of therapeutic management in clinical practice, medico-economic studies and improvement of a nationwide registry.
Acknowledgements
The authors wish to acknowledge Didier Blanchard, Grégoire Rangé, Nicolas Danchin, Etienne Puymirat, and Martine Gilard for their support in data collection.
Conflict of interest statement
P. Motreff has received consulting fees from Biotronik, St. Jude Medical and Terumo. B. Lattuca has received research grants from Daiichi Sankyo and Fédération Française de Cardiologie, consulting fees from Daiichi Sankyo and Eli Lilly, and lecture fees from AstraZeneca. H. Benamer has received consulting fees from Edwards, General Electric and Terumo. P. Commeau has received consulting fees from Boston Scientific and Terumo. G. Cayla has received grants or lecture fees from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Boston, Biotronik, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Europa, Fédération Française de Cardiologie, Fondation Cœur & Recherche, Medtronic, MSD, Pfizer, Sanofi-Aventis and The Medicines Company. J. Monsegu has received consulting fees from Terumo. E. Puymirat has received speaker, board membership, and consulting fees from Amgen, AstraZeneca, Bayer, Daiichi Sankyo, Lilly, MSD, and Sanofi-Aventis. V. Auffret has received research grants from Abbott, Edwards Lifesciences, Medtronic, Biosensors, Terumo, and Boston Scientific. The other authors have no conflicts of interest to declare.

