
Abstract
Aims: The aim of this substudy was to investigate the correlation between fractional flow reserve (FFR) and diameter stenosis in patients with STEMI with and without left ventricular hypertrophy (LVH), and the influence of LVH on complete FFR-guided revascularisation versus culprit only, in terms of risk of clinical outcome.
Methods and results: In this DANAMI-3-PRIMULTI substudy, 279 patients with STEMI had cardiac magnetic resonance (CMR) imaging for assessment of left ventricular mass index. Ninety-six patients had FFR evaluation of a non-culprit lesion. Diameter stenosis of the non-culprit lesion was determined with two-dimensional quantitative coronary analysis. The diameter stenosis (56.9% vs 54.3%, p=0.38) and FFR value (0.83 vs 0.85, p=0.34) were significantly correlated in both groups (Spearman’s ρ=–0.40 and –0.41 without LVH and with LVH, respectively; p<0.001) but were not different between patients without and with LVH (p for interaction=0.87). FFR-guided complete revascularisation was associated with reduced risk of death, myocardial infarction or ischaemia-driven revascularisation both for patients without LVH (HR 0.42, 95% CI: 0.20-0.85) and for patients with LVH (HR 0.50, 95% CI: 0.17-1.47), with no interaction between the FFR-guided complete revascularisation and LVH (p for interaction=0.82).
Conclusions: LVH did not interact with the correlation between diameter stenosis and FFR and did not modify the impact of complete revascularisation on the occurrence of subsequent clinical events.
Introduction
Fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) has not been validated in patients with left ventricular hypertrophy (LVH). FFR is a pressure-derived index used to assess the functional importance of coronary stenoses. FFR is defined as the ratio of distal coronary pressure to aortic pressure during maximal hyperaemia. An FFR value of 0.80 or less is considered physiologically significant. FFR-guided PCI has been validated extensively in randomised clinical trials of single and multivessel disease1,2,3. Moreover, FFR-guided complete revascularisation is superior to medical treatment in patients with both stable coronary artery disease and ST-segment elevation myocardial infarction (STEMI)4,5,6.
The impact of LVH on FFR measures has not been investigated in any of these trials. Moreover, it is not known whether the current cut-off value for treatment applies in patients with LVH. Theoretically, a larger subtended myocardium is expected to contain a higher total capillary number, and thereby a decreased resistance to flow. This translates to greater hyperaemic flow, increasing the pressure drop across a stenotic vessel, thereby lowering FFR. On the other hand, in patients with LVH factors such as extravascular compression, increased LV pressures and microvascular dysfunction may result in increased microvascular resistance, reduced hyperaemic flow and a subsequently higher FFR value. The interplay between these factors and FFR is unknown. Furthermore, in severe cases of LVH, as in aortic stenosis, high intraventricular pressure in combination with (occasionally) low aortic pressure would be expected to skew FFR towards higher values independent of stenosis severity. In this study however, we only investigated LVH in patients with no valvular disease. In this substudy of DANAMI in patients with STEMI and additional lesions in non-culprit arteries, the utility of FFR was investigated in the presence of LVH to the extent which is found in a representative STEMI population. The prevalence of LVH in patients with STEMI has been reported to be 24% and to be associated with a higher risk of all-cause mortality and development of heart failure7. In patients with stable coronary disease, the prevalence of LVH has been reported to be in the range of 16-50%8,9,10. Therefore, it is important to investigate whether FFR-guided PCI in patients with STEMI is influenced by the presence of LVH and thereby affects clinical outcome compared to patients without LVH.
We investigated the correlation between the angiographically assessed diameter stenosis and FFR in STEMI patients with and without LVH and assessed the interaction of LVH with clinical outcome (composite of all-cause mortality, myocardial infarction [MI] or ischaemia-driven revascularisation) in patients receiving culprit-only versus complete revascularisation. We hypothesised that patients with LVH, on average, had lower FFR at any given diameter stenosis, compared to patients without LVH.
Methods
STUDY POPULATION
This is a substudy of the DANAMI-3-PRIMULTI trial4, which was part of the DANAMI-3 trial programme (clinicaltrials.gov identifier: NCT01435408)11. DANAMI-3 consisted of three different multicentre, randomised trials4,12,13. DANAMI-3-PRIMULTI investigated the effect of culprit-only versus FFR-guided complete revascularisation, on a composite of all-cause mortality, MI or ischaemia-driven revascularisation in patients with STEMI4. Out of 627 cases in DANAMI-3-PRIMULTI, 314 were randomised to full revascularisation. As LVH was identified with cardiac magnetic resonance (CMR) imaging, we included only patients who had an index CMR carried out. CMR was only carried out in patients included in DANAMI-3-PRIMULTI at Rigshospitalet, Copenhagen, Denmark. Patients were divided into two groups according to the presence of LVH. Enrolment was from March 2011 to February 2014.
CORONARY ANGIOGRAPHY AND FFR
The culprit lesion was treated with primary PCI in all patients. Patients randomised to complete revascularisation underwent a second procedure with FFR-guided PCI of all lesions deemed suitable for PCI (angiographic diameter stenosis >50% in coronary artery branches with diameters ≥2 mm). The second procedure was performed at least 48 hours after the index procedure, but before discharge. FFR was not mandated in cases with a visually assessed diameter stenosis >90%. Intracoronary pressures were measured with a pressure wire (Abbott, Minneapolis, MN, USA). Hyperaemia was induced with intravenous adenosine infusion at a rate of 140 μg/kg/min. FFR was assessed as the lowest recorded value during two minutes of continuous infusion.
CARDIAC MAGNETIC RESONANCE
The CMR protocol has been described in detail elsewhere7. Briefly, CMR was performed during index admission following primary PCI, using a 1.5 Tesla scanner (Avanto™; Siemens, Erlangen, Germany). Images were analysed by two independent observers, blinded to all clinical data, using Circle Cardiovascular Imaging Inc. (Calgary, Alberta, Canada). LV mass was measured from standard ECG-triggered balanced steady-state free-precession cine images. Endocardial and epicardial contours were traced manually, with the papillary muscles included in the ventricular cavity. Body surface area was calculated using the DuBois formula. LVH was defined as left ventricular mass indexed for body surface area (LVMi) >77 g/m2 for men and 67 g/m2 for women, based on CMR data from 44 healthy subjects7.
QUANTITATIVE CORONARY ANALYSIS
Two-dimensional QCA was performed offline using QAngio® XA 7.3.82.0 (Medis medical imaging systems, Leiden, the Netherlands). The contrast-filled guide catheter was used as a distance calibration standard. QCA was performed by two independent investigators. Angiographic views with optimal stenosis delineation, contrast filling and least degree of foreshortening were chosen. Measurements were performed on end-diastolic frames. Manual correction of edge detection and reference diameter was carried out whenever necessary. QCA parameters were diameter stenosis, lesion length and area stenosis.
STATISTICAL ANALYSIS
Normality of data was evaluated with histograms. Differences in continuous variables were analysed using the Student’s t-test or Mann-Whitney U test when data were not normally distributed. Differences between proportions were assessed with the χ2 test or Fisher’s exact test. Spearman’s rank correlation coefficient was used to assess the correlation between diameter stenosis and FFR in each group. The interaction between LVH on the correlation between diameter stenosis and FFR was evaluated with an analysis of covariance (ANCOVA) model. Linear regression was used to assess the association between FFR and indexed left ventricular mass when corrected for diameter stenosis. We used Cox regression analysis to calculate hazard ratios for the primary outcome. The assumption of proportionality of hazards was evaluated with partial residual plots (Schoenfeld residuals test). Evaluation of the assumption of linearity was not relevant as no continuous variables were included in the model. Interaction between the prognostic implications of treatment allocation and the presence of LVH was tested in the Cox model. We used cumulative incidence rate curves to show differences between groups according to randomised treatment and LVH. Statistical analyses were carried out using SPSS Statistics, Version 22 (IBM Corp., Armonk, NY, USA). We considered p-values <0.05 to be significant.
Results
BASELINE CHARACTERISTICS
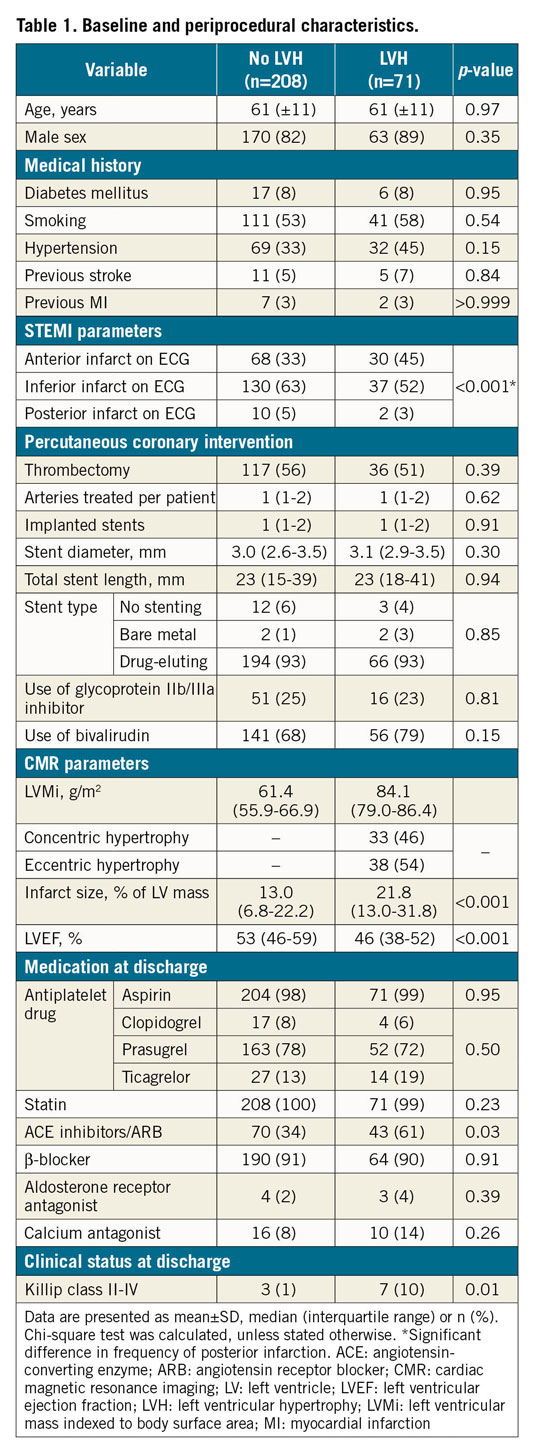
All patients with available index CMR were included in the study and evaluated for long-term outcome (n=279) (Figure 1). Of these, 71 (25%) patients had LVH and 208 (75%) had normal left ventricular mass (LVM). For the comparison between FFR and diameter stenosis at lesion level in patients with and without LVH, all patients with at least one FFR measurement and index CMR were included, totalling 96 of the 314 cases randomised to FFR-guided full revascularisation (Figure 1). The discrepancy between the number of cases with an FFR measurement (n=184) and the number of randomised cases (n=314) is explained in Figure 1. Of the 96 cases, 25 had LVH and 71 had normal LVM, corresponding to 34 and 100 lesions, respectively. Patients with and without LVH differed significantly in the frequency of posterior infarction, infarct size, left ventricular ejection fraction (LVEF) and Killip class at discharge. All baseline characteristics are listed in Table 1.
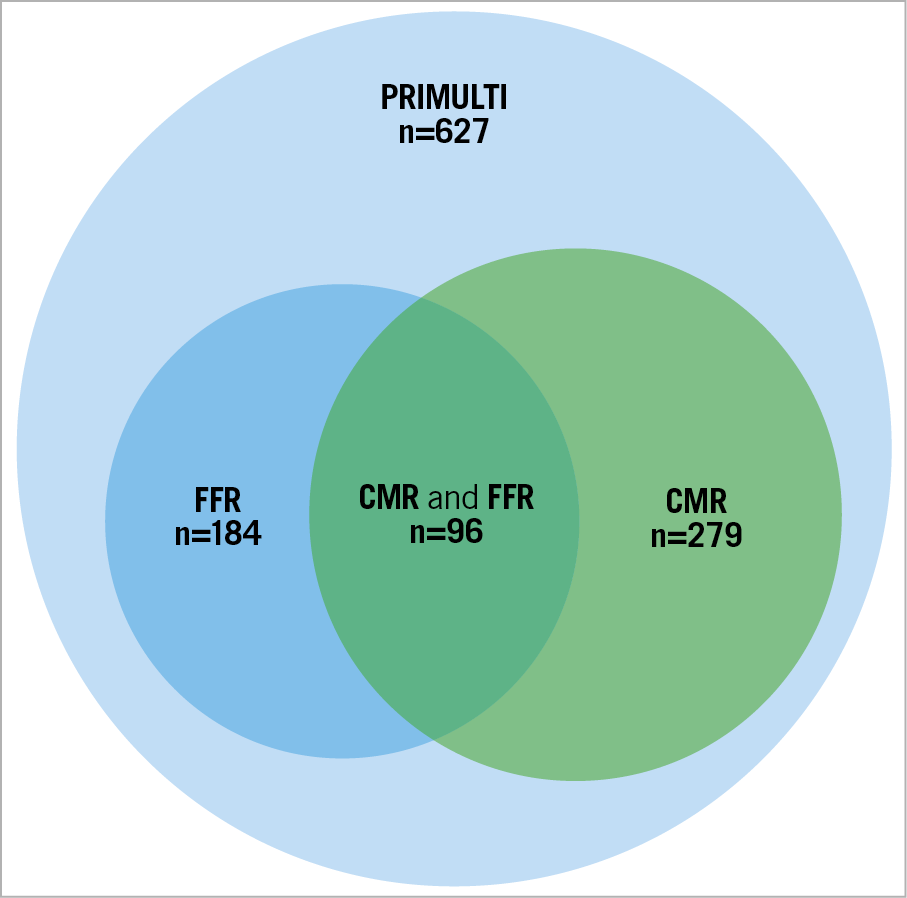
Figure 1. Venn diagram illustrating how data were obtained. FFR was measured in 184 of the 314 cases randomised to full FFR-guided revascularisation. In 81 cases, FFR was not measured due to diameter stenosis >90%25. In 35 cases, FFR data were not available as the procedure was performed at another site. In the remaining 14 cases, FFR was not measured due to technical issues (n=8), periprocedural complications (n=3), logistical and other reasons (n=3). CMR was performed in 279 cases. FFR and CMR were performed in 96 of the total 314 cases.
DIAMETER STENOSIS, INDEXED LEFT VENTRICULAR MASS AND FFR
There was no difference in the QCA parameters (diameter stenosis, area stenosis, lesion length) and FFR between groups (Table 2). The distributions of lesions in the major coronary branches were comparable. Differences in median, interquartile range, minimum and maximum values for diameter stenosis and FFR are shown in box plots (Figure 2). Diameter stenosis was significantly associated with FFR in both groups (Spearman’s ρ=–0.40 and –0.41 without LVH and with LVH, respectively; p<0.001 for both groups). LVH showed no interaction with this correlation in an ANCOVA model (p=0.87) (Figure 3). LVMi, as a continuous variable, was not significantly associated with FFR when corrected for diameter stenosis (β-coefficient 0.18, p=0.054). The correlation between area stenosis and FFR was comparable to that of diameter stenosis and FFR (Spearman’s ρ=0.40 for no LVH and 0.41 for LVH, p<0.001 and p=0.016, respectively).
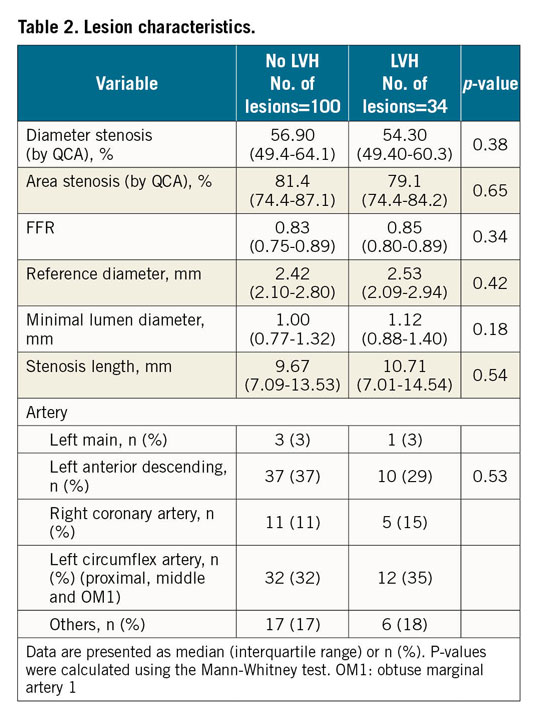
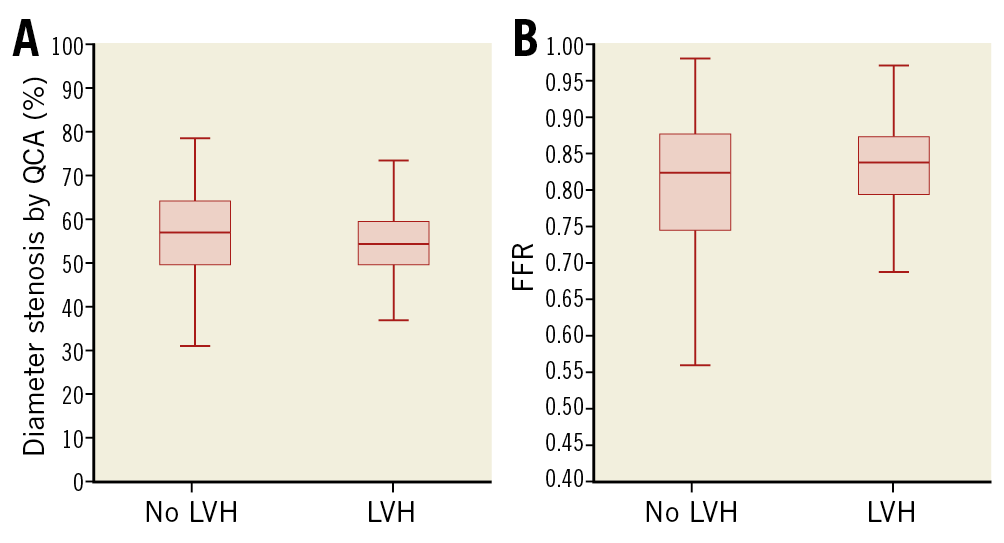
Figure 2. Diameter stenosis and FFR in patients with and without LVH. Box plots showing the median, interquartile range, minimum and maximum values for diameter stenosis (A) and FFR (B). There were no significant differences between groups for either variable.
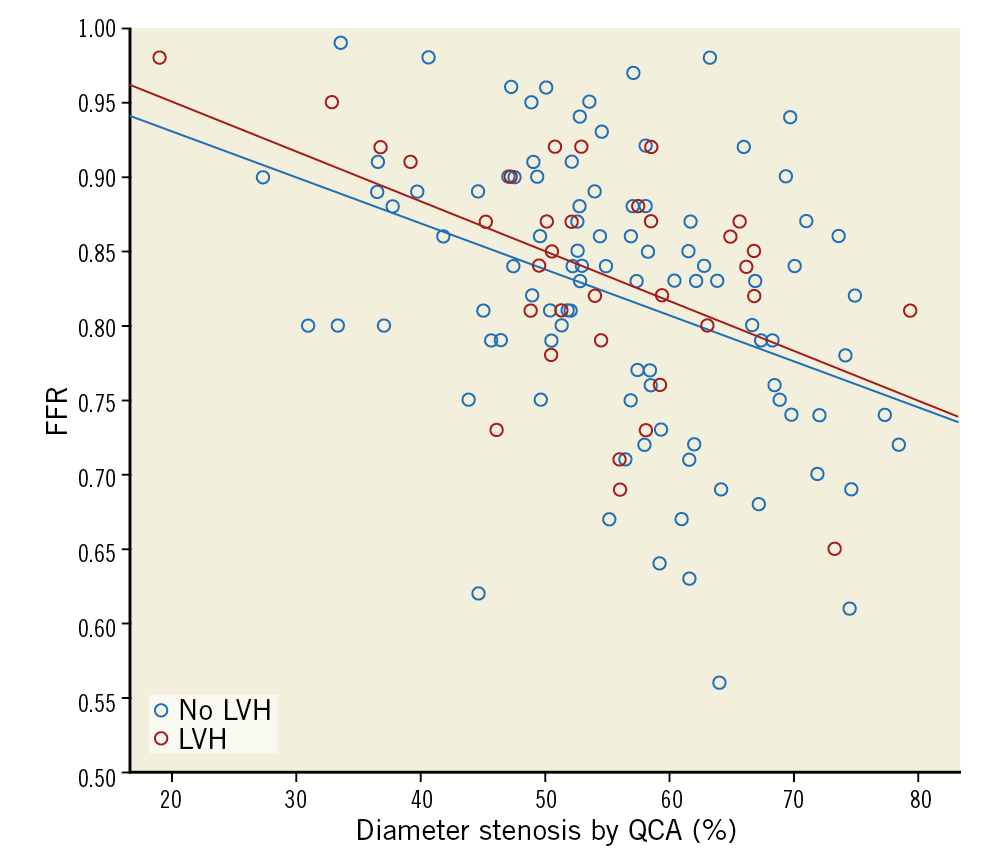
Figure 3. Correlation between diameter stenosis and FFR in patients with and without LVH. Trendlines for each group are shown. Trendlines were not significantly different when analysed in an ANCOVA model (p for interaction=0.87).
OUTCOME ANALYSIS
Median follow-up time was 23.4 months (interquartile range 16.5-33.0 months). In the present patient population, the hazard ratio (HR) was 0.44 (95% CI: 0.24-0.80, p=0.007) for the primary outcome in patients who were randomised to FFR-guided complete revascularisation, which is in line with the findings of the original study4. Figure 4 shows cumulative incidence rate curves according to LVH and treatment strategy (FFR-guided complete revascularisation and culprit only). The HR was 0.50 (95% CI: 0.17-1.47) for patients with LVH and 0.42 (95% CI: 0.20-0.85) for patients without LVH, favouring FFR-guided complete revascularisation, with no interaction between revascularisation strategy and the presence of LVH (p=0.82).
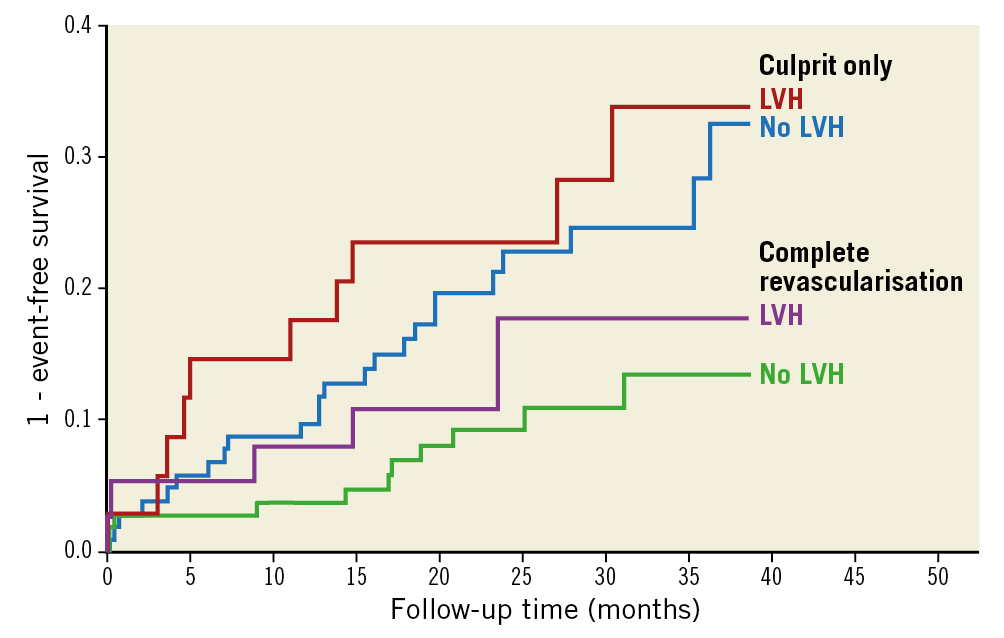
Figure 4. Cumulative incidence rate curves of patients randomised to treatment of culprit-only or complete revascularisation. Patients are grouped according to presence of LVH. There was a significant difference between treatment groups (p=0.007), but no difference between treatment groups when grouped according to LVH (p for interaction=0.82).
Discussion
FINDINGS
The main findings of this DANAMI-3-PRIMULTI substudy were that LVH did not influence the correlation between FFR and diameter stenosis as assessed by QCA, nor the risk of clinical outcome following FFR-guided complete revascularisation versus culprit only in patients with STEMI, although LVH in itself appears to impair prognosis, as demonstrated in another DANAMI-3 substudy7. Thus, from a clinical perspective, the presence of LVH should not affect the interpretation and clinical use of FFR in stable non-culprit territories in patients with STEMI. The HR of 0.44 (95% CI: 0.24-0.80, p=0.007) in this subpopulation was comparable to that reported for the entire DANAMI-3-PRIMULTI population (HR 0.56, 95% CI: 0.38-0.83, p=0.004)4. This does not preclude the possibility of a different (higher) cut-off value for treatment when LVH is present, but perhaps suggests that it does not differ much from the current cut-off value.
POTENTIAL PATHOPHYSIOLOGICAL MECHANISMS
Haemodynamic theory suggests that a large myocardium subtended by a stenotic vessel imposes a lower FFR at a given stenosis severity when compared to a smaller myocardium14. Theoretically, this would mean that, if the subtended myocardium has a mass of 50 g and FFR in the stenotic vessel is 0.80, the same myocardium should, at an increase in mass to for example 60 g, yield a lower FFR of, for example, 0.70 (Figure 5). An inverse relationship between the amount of myocardium subtended by a given stenotic vessel and FFR has been suggested15. In the study by Leone and colleagues, data were in accordance with theory, but patients with acute coronary syndrome and severe LVH were excluded15. This limits the comparability to our study as differences in size of subtended myocardium may still be within the physiological range in their population. Another study of 84 patients compared correlations of diameter stenosis and FFR in matched vessels in patients with normal and increased LV mass, measured using contrast ventriculography16. They found no difference in FFR and no interaction of LVH on the relationship between diameter stenosis and FFR, which is in line with the findings in our study. Our study adds to previous observations, as LVM was measured using CMR and provides information regarding clinical outcome following revascularisation strategy in patients with LVH. The finding that the presence of LVH does not impact on the correlation between diameter stenosis and FFR can be explained by several factors. 1) LVH is associated with microvascular dysfunction and decreased coronary flow reserve17,18,19. 2) LVH is often accompanied by diastolic dysfunction, with increased levels of extravascular compression of the intramyocardial microcirculation. 3) The prevalence of smoking and diabetes, which both promote macrovascular disease but also impair microcirculatory function by decreasing nitric oxide production and bioavailability20,21, is considerable. All these factors could counteract the effect of an increase in functionally subtended myocardium. In other words, the absence of differing results may indicate that the influence of increased myocardium size on FFR is counterbalanced by decreased coronary flow reserve (microvascular dysfunction) and increased extravascular resistance. Consequently, existing data, including the results from the present study, show that the presence of LVH does not influence the correlation between the FFR value and diameter stenosis severity in patients with STEMI.
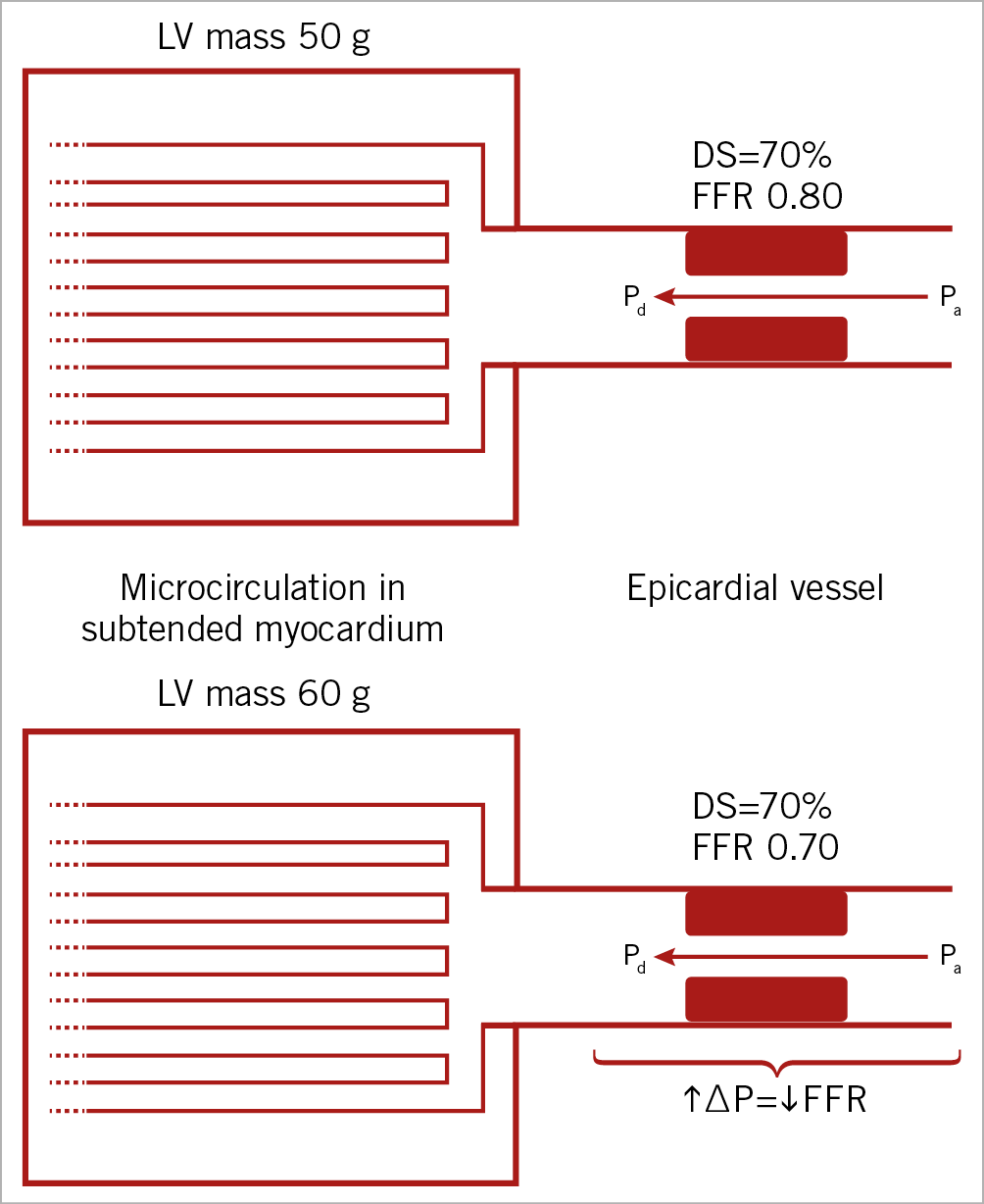
Figure 5. Simplified theoretical relationship between size of subtended myocardium and FFR at a given stenosis severity. An increase in subtended myocardial mass at an arbitrarily chosen diameter stenosis is expected to result in a lower FFR. DS: diameter stenosis; FFR: fractional flow reserve; LV: left ventricle; Pa: aortic pressure; Pd: distal coronary pressure
FUTURE STUDIES
One way to clarify how LVH modulates FFR would be to measure i) coronary flow reserve to assess microvascular dysfunction, ii) absolute volumetric flow indexed to LVM to assess baseline vasodilation (although capillary density would be a confounder), and iii) index of microvascular resistance and left ventricular end-diastolic pressure to assess total vascular resistance including the extravascular compressive component. In endurance athletes, physiological LVH is characterised by conserved capillary density, representing a “balanced” model of increased subtended myocardium size. A comprehensive physiological work-up as outlined above may yield interesting findings, if performed in LVMi-matched subjects with pathological LVH and exercise-induced LVH. In addition, pathological LVH from different patient categories (for example, hypertension, aortic stenosis) may be different and should be examined. However, in the present setting LVH did not indicate a need for change in decision making, as it did not affect clinical outcome.
Limitations
This is a retrospective study and a non-pre-specified sub-analysis. CMR was only performed at one site (Rigshospitalet). Thus, CMR data were available for only 279 of the total 627 (45%) patients in DANAMI-3-PRIMULTI. However, the HR for clinical outcomes was comparable to that of the original study4. STEMI causes myocardial oedema in the infarct area, which could have influenced the CMR estimation of LVM and thus LVH7. Patients with LVH had a significantly larger infarct size, which may have influenced FFR in non-culprit arteries. Ideally, QCA should have been performed by a core laboratory. There was a weak correlation between diameter stenosis and FFR. However, this is not unique to our study and has been shown previously in several studies22,23. An effect of LVH on the relation between diameter stenosis and FFR (type 2 error) cannot be excluded as the number of cases with data on both CMR and FFR was small. The number of lesions for analysis was limited because FFR was not measured in the culprit-only arm of DANAMI-3-PRIMULTI. Moreover, the lack of FFR measurement in vessels with diameter stenosis >90% constrained FFR values to a narrow range (Table 2). This probably made it harder to detect differences in the correlation between diameter stenosis and FFR, which may have been more pronounced at greater stenosis severity. However, any potential difference between groups was probably small as clinical outcome was not affected by LVH, although this may also be due to a negligible impact of FFR values near 0.80 on prognosis24. Non-hyperaemic pressure measurements were not available for analysis. Finally, an effect of LVH on FFR in severe hypertrophic patients such as those with aortic stenosis cannot be excluded based on the findings in this study.
Conclusions
In the DANAMI-3-PRIMULTI population of patients with STEMI, the presence of mild to moderate LVH did not influence the correlation between FFR value and diameter stenosis. The advantage of FFR-guided complete revascularisation compared to culprit lesion only was similar between patients with and without LVH.
|
Impact on daily practice FFR-guided PCI has become widespread but has not been validated in patients with left ventricular hypertrophy (LVH). In this retrospective study of the DANAMI-3-PRIMULTI trial, we showed that LVH (measured with cardiac magnetic resonance), in patients with STEMI randomised to complete FFR-guided or culprit-only PCI, does not interact with the correlation between diameter stenosis and FFR. Importantly, the advantage of FFR-guided complete revascularisation compared to culprit lesion only was similar between patients with LVH and without LVH. |
Funding
This study was funded by The Heart Center, Rigshospitalet – Copenhagen University Hospital, Copenhagen, Denmark.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

