A 79-year-old woman was admitted with dyspnoea NYHA Class III to IV. Five years previously, she had undergone surgical aortic valve replacement (Mitroflow® 19 mm; Sorin S.p.A., Milan, Italy). Transthoracic echocardiography revealed a degenerated bioprosthesis (area 225.7 mm2, perimeter 53.4 mm, area and perimeter derived diameter 17 mm; Figure 1A, Figure 1B) with a severe stenosis (aortic valve area 0.63 cm2), a severe transvalvular and mild paravalvular regurgitation, and a severely reduced left ventricular ejection fraction. Because of her symptomatic valvular heart disease and the high perioperative risk (logistic EuroSCORE 18%), she was referred for transfemoral transcatheter aortic valve-in-valve implantation. Given the specific design of the Mitroflow® valve with its pericardial leaflets mounted outside the stent and the specific anatomy of the patient with a shallow sinus aortae and a low origin of both coronary arteries, the risk of ostial encroachment was deemed substantial. Due to its small size and the unique characteristics of retrievability and resheathability, the decision was made to use a Portico® 23 mm valve (St. Jude Medical Inc., St. Paul, MN, USA; Figure 1C). After the intervention, there was a decrease of the mean aortic gradient from 29 mmHg to 19 mmHg, an increase of the diastolic pressure from 30 mmHg to 51 mmHg, a decrease of the left ventricular filling pressure from 26 mmHg to 21 mmHg (Figure 1D, Figure 1E), an increase of the aortic regurgitation index from 3.5 to 22, and a reduction of aortic regurgitation from grade 3 to 1. The further clinical course was uneventful and the patient was discharged two days later. Three months after the intervention, symptoms improved to NYHA Class II, and transthoracic echocardiography exhibited an unchanged severely impaired left ventricular ejection fraction but a mean aortic gradient of only 18 mmHg with minimal paravalvular aortic regurgitation.
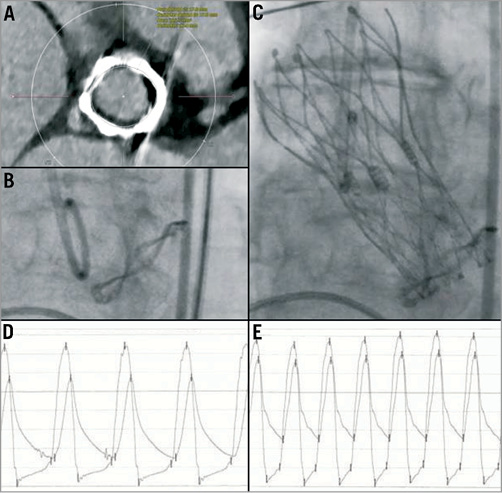
Figure 1. A) Analysis of the degenerated Mitroflow® valve size using EKG-triggered computed tomography and the 3mensio® software. B) Pre-interventional angiography showing the characteristic sewing ring of the Mitroflow® valve in the aortic root. C) Post-interventional angiography of the aortic root with a Portico® transcatheter aortic valve placed in the degenerated Mitroflow® valve. D) Pre-interventional simultaneous measurement of aortic and left ventricular pressures with an aortic regurgitation index of 3.5. E) Post-interventional double pressure haemodynamic tracing with an aortic regurgitation index of 22.
Conflict of interest statement
G. Manoharan is proctor for the manufacturer of the valve, St. Jude Medical. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Pre-intervention.
Moving image 2. Post-intervention.

