Abstract
Aims: Intravascular ultrasound (IVUS) is a proven and safe imaging modality used to guide percutaneous coronary intervention (PCI). The Volcano VIBE™ RX Vascular Imaging Balloon Catheter is a novel rapid exchange, 0.014” wire-compatible multi-lumen conventional balloon catheter modified with the addition of an IVUS transducer proximal to the balloon, delivered via a standard 6 Fr sheath. We sought to evaluate the safety, balloon performance, and image quality of the VIBE™ RX in patients scheduled for coronary intervention.
Methods and results: Patients aged >21 and <85 years with single or multivessel coronary disease scheduled for PCI due to coronary ischaemic symptoms were included. Those with angiographic features that precluded the safe or informative use of the device were excluded. Twenty-nine patients having angiography because of ischaemic symptoms underwent 44 VIBE RX imaging runs, with balloon dilation in 20. Successful device deployment was achieved in all but one patient. All images were adequate and reproducible. One patient had a non-ST-elevation MI felt to be due to the complexity of the procedure rather than directly related to the VIBE™ RX.
Conclusions: The study demonstrated the safety and effectiveness of the VIBE™ RX for its intended purpose with minimal failure rate and no directly related complications.
Introduction
Coronary angiography remains the primary imaging modality used to assess the severity of coronary artery disease. However, despite such broad application, angiography primarily provides a silhouette of the contrast-filled vessel lumen albeit with considerable variability in inter- and intra-observer interpretation1,2. Moreover, although able to determine the presence of extensive calcification, angiography is unable to determine the precise nature of coronary atheroma and therefore has a number of important limitations. Furthermore, in the era of percutaneous coronary intervention (PCI), assessing stent apposition to the vessel wall, establishing the nature of in-stent restenosis and assessing for intimal dissection have, among others, become important requirements3-5.
Intravascular ultrasound (IVUS) has evolved as a safe, robust and reproducible imaging modality complementary to conventional angiography6,7. IVUS is capable of producing high quality images with an axial resolution of approximately 100 microns and lateral resolution of up to 250 microns allowing interrogation of the vessel wall through to the tunica media. Both axial resolution and lateral resolution are important determinants of spatial resolution and determined largely by the frequency of the imaging transducer8. Consequently, IVUS technology has developed several niche applications such as assessment of ambiguous disease and as a tool to guide complex PCI procedures9,10. Furthermore, recent technological advances have permitted assessment of diastolic coronary plaque deformability, potentially allowing virtual histological identification of vulnerable plaque11,12.
Currently IVUS is employed as a stand-alone dedicated imaging transducer incorporated into a rapid exchange catheter, thereby requiring a number of catheter exchanges, particularly if serial imaging is to be undertaken before and after balloon (or stent) deployment. Consequently, there is increased procedure time, radiation exposure and potential for thrombotic complications.
These challenges have led to the development of the VIBE™ RX catheter (Volcano Corp., San Diego, CA, USA). This unique hybrid design incorporates both an IVUS transducer and a semi-compliant balloon, allowing seamless lesion assessment, preparation/treatment and reassessment with a single catheter. Although similar catheter designs were briefly available historically, none has been evaluated systematically in the clinical setting. The VIBE-NZ (Volcano IVUS-guided Balloon Evaluation, New Zealand) study was designed to assess the safety and performance of this device for assessment and treatment of coronary artery stenoses.
Methods
DEVICE DESCRIPTION
The VIBE™ RX catheter is a 150 cm rapid exchange semi-compliant balloon catheter with an integral IVUS transducer positioned/sited proximal to the balloon (Figure 1, Figure 2, Figure 3). The multi-lumen catheter design allows for balloon inflation, IVUS transducer connection and advancement of the catheter over a 0.014” guidewire. A Y-shaped adaptor is attached to the proximal end of the catheter shaft to provide access to the inflation lumen and to carry the (IVUS) cable wire bundle. A hydrophilic polymer coating (GlyDx®) is applied to the external surface of the distal portion of the catheter from tip to exit of the rapid exchange port to aid deliverability. Due to the hybrid design, the system has a maximal external diameter of 3.5 Fr, which is slightly greater than the 3.2 Fr iCross™ (Boston Scientific Corp., Natick, MA, USA) and the Eagle Eye® Gold (Volcano Corp.) IVUS catheters, although it remains compatible with 6 Fr (≥0.066” internal diameter) guide catheters.
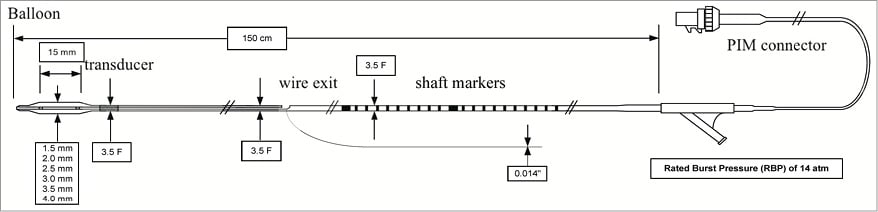
Figure 1. Schematic diagram of the VIBE™ RX IVUS balloon catheter system. Note the patient interface module (PIM) cable bundle exiting from the Y-connector.
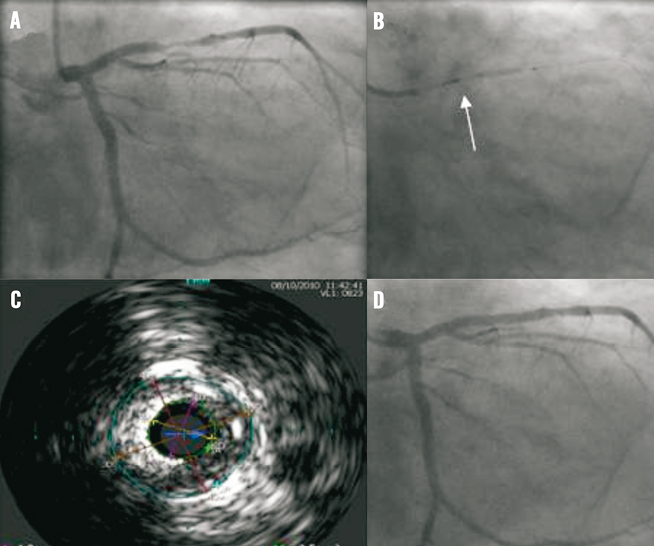
Figure 2. VIBE™ RX catheter treating a mid left anterior descending artery stenosis. A) Note stenosis in mid left anterior descending artery. B) After vessel wiring, the VIBE™ RX catheter is used to predilate the stenosis. Note imaging transducer (arrow). C) IVUS allowed accurate assessment of vessel reference diameter aiding stent selection. D) Final angiographic result following deployment of a Promus Element 3.5×16 mm stent, post-dilated to 4 mm proximally.
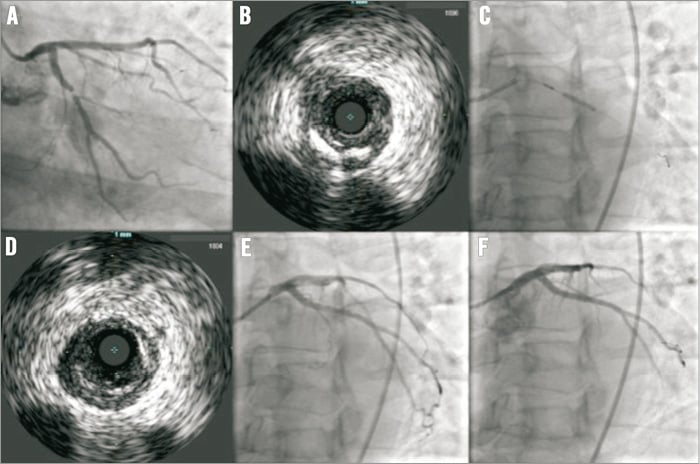
Figure 3. VIBE™ RX catheter treating a left circumflex artery. A) Severe proximal circumflex stenosis at baseline. B) VIBE™ RX IVUS of the lesion before predilation, noting mild calcification. C) Predilation with a 2.5 mm VIBE™ RX balloon. D) IVUS of the lesion following predilation. E) Angiographic appearance after predilation. F) Final angiographic appearance after implantation of a Promus Element 3×12 mm stent post-dilated with a 3.5 mm non-compliant balloon.
The IVUS transducer is a cylindrical, solid-state, 64-element 20 MHz phased-array ultrasonic transducer with a 20 mm field of view (imaging diameter). The transducer is identical to that incorporated within the Volcano Eagle Eye® Platinum Catheter and is compatible with the Volcano s5™ and s5i™ Imaging Systems running software version 3.0.2 or higher. The transducer is capable of producing both two-dimensional greyscale and virtual histology imaging, potentially therefore able to aid identification of vulnerable plaque. Graduated incremental withdrawal of the transducer at 0.5 mm/sec is possible by connection to the Volcano TrackBack II or R-100 pullback systems.
The catheter has two radiopaque markers sited at either end of the balloon to aid positioning within the target lesion. For the VIBE-RX study, six models of the balloon were available, in ½ mm increments ranging from 1.5 to 4.0 mm diameter, all 15 mm in length. Nominal inflation pressure is 8 atm with rated burst at 14 atm.
STUDY OVERVIEW AND PATIENT POPULATION
The study was a prospective, multicentre, non-randomised single-arm trial designed to evaluate the feasibility and safety of the VIBE™ RX IVUS-guided balloon catheter in patients scheduled for PCI. Between May and October 2010, two centres in New Zealand enrolled 29 patients with symptomatic ischaemic heart disease attributable to coronary artery disease. The study was approved by the Northern X Research Ethics Committee (Ministry of Health, New Zealand).
Patients >21 and <85 years of age who were willing to participate and able to provide informed consent were included. Absence of pregnancy was ascertained in women of child-bearing age. Patients with any myocardial infarction (MI) within five days of the procedure, with a creatine kinase (CK) level of ≥2x the upper limit of normal or persistently abnormal creatine kinase-myocardial band isoenzyme (CK-MB) fraction at the time of the procedure were excluded, as were all patients with anticipated further revascularisation within 30 days of the index procedure. Other exclusions included clinical heart failure or left ventricular ejection fraction <30%, significant renal insufficiency (estimated creatinine clearance of <50 ml/min), significant valvular heart disease or a cerebrovascular accident (CVA)/transient ischaemic attack (TIA) within the past 60 days. Those who were unable to take dual antiplatelet agents or who had significant blood dyscrasia and those with known contrast allergy were excluded. Angiographic exclusion criteria included ostial or bifurcation lesions, total occlusions, severe calcification or tortuosity and vessels <2 mm and >4 mm in diameter either by visual assessment or by quantitative coronary angiography (QCA).
INTERVENTIONAL PROCEDURE
Prior to the procedure, all patients received aspirin (minimum 75 mg daily, commencing at least 24 hours prior to the procedure and continued indefinitely) and clopidogrel (minimum 300 mg loading dose within 24 hours prior to the procedure and then 75 mg daily for a minimum of one month depending on stent type). During the procedure, heparin was administered to maintain an activated clotting time of >300 sec or >250 sec if a glycoprotein IIb/IIIa inhibitor was administered. Administration of 100-200 mcg intracoronary nitroglycerine was required prior to each IVUS run and before/after balloon inflation.
Route of arterial access (i.e., femoral vs. radial) and coronary stent choice was left to the operator’s discretion, as were the number of VIBE™ RX IVUS runs performed per patient and whether the balloon was used only for predilation or also for post-dilation. Use of the balloon catheter as a simple IVUS without balloon inflation was also permitted.
PATIENT FOLLOW-UP
Patient follow-up was performed at 30 days by telephone consultation or office visit to the investigational site where the index procedure was performed.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoints of interest were:
1. Device safety: event-free survival at 30 days from a combination of cardiovascular death, non-fatal myocardial infarction (MI), and target vessel revascularisation (TVR).
2. Device performance endpoints:
a) primary: percentage change in minimum lumen diameter post-balloon dilatation, assessed by quantitative coronary angiography (QCA);
b) secondary: successful balloon catheter delivery to the target lesion, dilatation of the lesion using the study device, and successful catheter retrieval.
3. Imaging effectiveness: defined as the reproducibility of image measurements and intra- and inter-observer agreement in overall procedural and pre- and post-balloon dilatation measures of lumen and external elastic membrane (EEM) cross-sectional area (CSA) on QCA. Intra- and inter-observer agreement was calculated with measurements from two independent core lab image specialists.
For the purpose of the study, MACE was defined as a composite of cardiovascular death, non-fatal myocardial infarction (MI, Q-wave and non-Q-wave), and target vessel revascularisation (TVR). Q-wave MI was defined as chest pain or other acute symptoms consistent with myocardial ischaemia associated with new pathological Q-wave formation in two or more contiguous ECG leads. New pathological Q-wave formation in two or more contiguous leads without ischaemic symptoms but in the presence of elevated cardiac enzymes was also considered to be a Q-wave MI. A non-Q-wave MI was defined as an elevated CK-MB fraction ≥3x upper limit of normal in the absence of new pathological Q-waves. TVR was defined as any percutaneous intervention or bypass surgery performed on the target vessel at any point during follow-up after the index procedure.
SAMPLE SIZE AND STATISTICAL ANALYSES
The VIBE-NZ study was designed to minimise the number of subjects exposed to the novel IVUS-balloon hybrid system whilst still providing adequate data about catheter performance, feasibility and safety. A sample size of 30 was deemed sufficient to meet these objectives. As this was a clinical feasibility study, the sample size was not powered for statistical significance comparisons of outcome measure.
All analyses were based on an intention-to-treat principle. Discrete variables are expressed as frequencies and percentages with continuous data expressed as mean±standard deviation or median and range for parametric and non-parametric data, respectively.
DATA COLLECTION AND CORE LABORATORY
All data were submitted to a central data co-ordinating facility (Volcano Corporation, San Diego, CA, USA), which also monitored the conduct of the trial. Major adverse events were reviewed independently by the site investigator, Volcano’s Chief Medical Officer and by the Northern X Regional Ethics Committee to determine any relationship to the study device. In the case of disagreement, an independent clinical events committee was available for final adjudication. All ECGs, coronary angiograms and IVUS images were reviewed by an independent core laboratory (Imaging Core Laboratory, St Luke’s Hospital, Kansas City, MO, USA) and adjudicated by two independent reviewers.
Results
PATIENT DEMOGRAPHICS
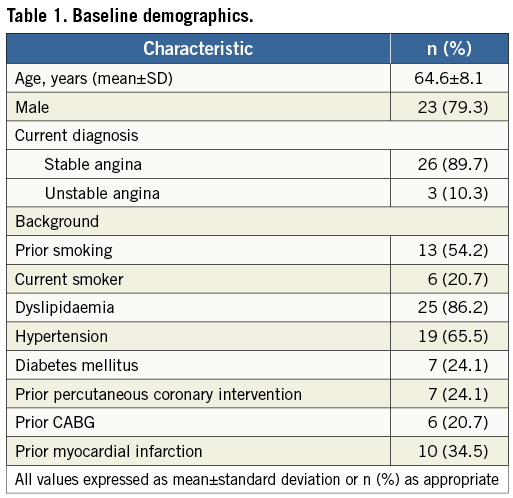
Patient demographics are summarised in Table 1. Of the 29 patients recruited, the majority were male (79.3%) with a mean age of 64.6 years. The vast majority had stable angina (88.9%), the remainder having presented within the previous 72 hours with an acute coronary syndrome. All received aspirin and clopidogrel.
LESION CHARACTERISTICS AND PROCEDURAL DATA
Lesion characteristics are summarised in Table 2. The majority of lesions interrogated involved the left anterior descending coronary artery and were mostly American College of Cardiology/American Heart Association class B1 or B2. The overall median vessel reference diameter was 3 mm and median lesion length 24 mm. In the study, a total of 30 catheters were used as an additional catheter was used in one patient for multivessel disease interrogation. The majority were 2.0 and 2.5 mm balloons (10 and 15, respectively), with two each of the 1.5 and 3.5 mm balloons being used, one 3.0 mm balloon and none of the 4.0 mm size.
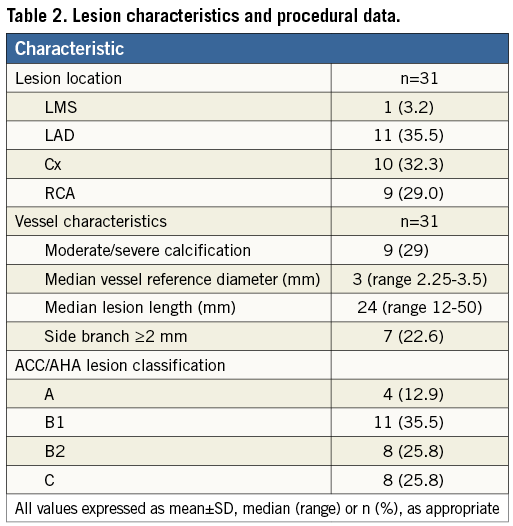
Overall, 47 imaging runs were completed, 15 in a native coronary artery, three within a previously sited stent, nine post balloon dilatation and 20 post stent deployment. In total, 20 balloon dilatations were performed, 17 in a native coronary artery and three within a previously sited stent.
PROCEDURE OUTCOMES
ACUTE DEVICE SAFETY
The 30-day MACE rate in the study was 3.5%. This related entirely to one event in a patient who sustained a non-Q-wave MI prior to hospital discharge. The total CK level was 867 U/L (ref 30-180) with a CK-MB of 132 µg/L. This was considered a consequence of the PCI procedure, which had been complex, rather than related to the VIBE™ RX catheter. It did not delay his discharge. In addition, there were seven other serious adverse events (SAE) per protocol. Five of these were chest pain not considered to be cardiac by the managing physician, and two were chest pain and palpitations felt to be unrelated to the procedure or device.
CATHETER DELIVERABILITY AND RETRIEVAL
Of the sizes used (all but the 4 mm balloon), the device performance secondary endpoint of reach, inflate, deflate and retrieve was achieved in 95% of catheter runs. In one patient, it was not possible to pass the VIBE™ RX catheter successfully to the region of interest. In this patient, the catheter was unable to cross the lesion pre-treatment but, following stent deployment, a successful catheter pass was completed. The catheter was successfully retrieved in all cases.
BALLOON PERFORMANCE
Balloon performance was assessed in a sub-analysis of 15 patients where QCA data were available both pre- and post-balloon inflation. Balloon dilatation improved the mean luminal stenosis from 73.3±9.4% to 43.6±13.3%, p<0.0001. This represented a mean change in luminal stenosis of 29.7±11.4%. This was associated with a subjective improvement in Thrombolysis In Myocardial Infarction (TIMI) flow in the majority of cases where less than TIMI grade 3 flow was observed at baseline.
IMAGING EFFECTIVENESS
Imaging effectiveness was evaluated in 25 pullbacks (nine pre-balloon dilatation and 16 post-balloon dilatation), by comparing measurements of external elastic membrane cross-sectional area (EEM-CSA) and lumen CSA measured at the core laboratory by two independent specialists. Five images were randomly selected from each pullback, and the specialists analysed the images twice two weeks apart. Six Bland-Altman plots were developed, each listing the mean difference and the 95% confidence interval bands of the average difference. Image quality was considered excellent and the measurements were highly reproducible and within acceptable clinical bounds of agreement.
Discussion
In this first-in-human feasibility study of the Volcano VIBE™ RX catheter in a mixed cohort of patients with coronary artery disease, we were able to deliver the catheter successfully in 95% of cases. The balloon system was effective at reducing mean luminal stenosis by an average 29.7±11.4% assessed by QCA. In addition, the IVUS transducer proved to be an effective and reproducible imaging tool. Device safety was good with a 30-day MACE rate of 3.5% related to a single event, which was considered unrelated to the study device.
The combination of IVUS transducer and semi-compliant balloon on a single catheter did allow accurate evaluation and proved helpful in positioning of the balloon over the region of interest during lesion preparation. Importantly, the balloon system was not used for lesion predilatation in 1/3 lesions. In such cases, following acquisition of IVUS data, operators opted for either a direct stenting strategy or predilatation with a larger calibre non-compliant balloon.
However, the VIBE™ RX catheter potentially allowed for greater precision in stent size selection based on IVUS assessment rather than angiographic estimates and was particularly helpful in one case with tortuous anatomy where the region of interest was overlapped by other arteries in multiple views. In addition, the balloon system appeared to wrap well once fully deflated. In all cases where a second IVUS run was undertaken following stent deployment, the system tracked to the region of interest without resistance and in some cases was used for post-dilatation. Such versatility may have a particular advantage in smaller calibre vessels, especially when treated with bare metal stents, as IVUS-guided optimisation has previously been shown to reduce late lumen loss13.
Moreover, there was a potential time-saving advantage in those cases where the operator had considered pre- and post-balloon dilatation IVUS an important component of the PCI procedure. Notably, the system was simple to use by connection to the familiar Volcano interface. An important pitfall of some multipurpose devices is the presence of numerous ports and connections, particularly at the distal end, potentially making handling of the catheter cumbersome. We found this not to be the case and instead found that the VIBE™ RX catheter was practical, intuitive and agile to use.
Limitations
We noted some limitations using the first generation of the VIBE™ RX system. Firstly, the catheter has a similar crossing profile to other commercially available IVUS catheters, albeit with a longer monorail segment. Although this should allow greater transmission of force, the addition of a balloon proximal to the IVUS transducer may reduce tip flexibility: thus, negotiating tortuous, calcified anatomy is potentially more problematic. Secondly, once used for lesion preparation, following stent deployment, the balloon was frequently no longer of adequate calibre to be effective for post-dilatation. Thirdly, there is naturally apprehension in passing a used balloon – particularly if it is incompletely wrapped – through a recently deployed stent, for fear of strut disruption. This point is particularly apt in an era of great concern about longitudinal stent deformation14. We did not encounter such a problem in our small series.
The VIBE-NZ study itself has a number of important limitations. The study was a first-in-human feasibility study and therefore recruited only a small number of patients, many of whom had relatively simple non-calcified coronary stenoses in native vessels without significant tortuosity. The study was also a non-randomised, single-arm trial conducted in only two centres. It allowed for use of the VIBE™ RX catheter as a combined IVUS-balloon device or as a simple imaging catheter, limiting the number of patients where balloon performance could be assessed. Although the data from this study demonstrate the feasibility, safety and efficacy of the device, larger studies are required to establish a clear role for the VIBE™ RX catheter. In its present form, the VIBE™ RX system has a limited yet potentially useful application in current practice. One important future niche may be for evaluation and treatment of in-stent restenosis, particularly should the system evolve to one assembled around a drug-coated balloon. Similarly, a system combined with a short non-compliant balloon may allow for high-pressure stent or lesion expansion guided precisely by IVUS imaging.
Conclusions
The VIBE-NZ study investigated the feasibility, safety and efficacy of the VIBE™ RX IVUS-balloon catheter for the assessment, treatment and reassessment of coronary artery stenoses.
The study results demonstrate that the catheter system is safe and effective for its intended use and can be easily and successfully delivered and deployed without significant complications at thirty days.
Acknowledgements
The authors wish to thank Dr G. Armstrong and Dr A. Khan for their assistance in recruiting patients for this study.
Funding
Volcano Corporation, San Diego, CA, USA.
Conflict of interest statement
J. Ormiston serves as an advisory board member for Abbott Vascular and Boston Scientific. The other authors have no conflicts of interest to declare.

