Abstract
Aims: There remains significant concern about the long-term safety of drug-eluting stents (DES). However, bare metal stents (BMS) have been used safely for over two decades. There is therefore a pressing need to explore alternative strategies for reducing restenosis with BMS. This study was designed to examine whether IVUS-guided cutting balloon angioplasty (CBA) with BMS could convey similar restenosis rates to DES.
Methods and results: In the randomised REstenosis reDUction by Cutting balloon angioplasty Evaluation (REDUCE III) study, 521 patients were divided into four groups based on device and IVUS use before BMS (IVUS-CBA-BMS: 137 patients; Angio-CBA-BMS: 123; IVUS-BA-BMS: 142; and Angio-BA-BMS: 119). At follow-up, the IVUS-CBA-BMS group had a significantly lower restenosis rate (6.6%) than the other groups (p=0.016). We performed a quantitative coronary angiography (QCA) based matched comparison between an IVUS-guided CBA-BMS strategy (REDUCE III) and a DES strategy (Rapamycin-Eluting-Stent Evaluation At Rotterdam Cardiology Hospital, the RESEARCH study). We matched the presence of diabetes, vessel size, and lesion severity by QCA. Restenosis (>50% diameter stenosis at follow-up) and target vessel revascularisation (TVR) were examined. QCA-matched comparison resulted in 120-paired lesions. While acute gain was significantly greater in IVUS-CBA-BMS than DES (1.65±0.41 mm vs. 1.28±0.57 mm, p=0.001), late loss was significantly less with DES than with IVUS-CBA-BMS (0.03±0.42 mm vs. 0.80±0.47 mm, p=0.001). However, no difference was found in restenosis rates (IVUS-CBA-BMS: 6.6% vs. DES: 5.0%, p=0.582) and TVR (6.6% and 6.6%, respectively).
Conclusions: An IVUS-guided CBA-BMS strategy yielded restenosis rates similar to those achieved by DES and provided an effective alternative to the use of DES.
Abbreviations
CBA: cutting balloon angioplasty
BMS: bare metal stent
DES: drug-eluting stent
IVUS: intravascular ultrasound
QCA: quantitative coronary angiography
Introduction
While drug eluting stents (DES) represent a significant advance in coronary intervention, there remain serious concerns about their long-term efficacy and safety1-5. Although major multicentre randomised studies including RAVEL, SIRIUS and TAXUS have suggested that DES could drastically reduce six to nine month restenosis rates to 0-8.9%, there is a paucity of long-term follow-up and safety data beyond five years6-10. Reports from the RADAR project and others have warned that subacute stent thrombosis, late and very late stent thrombosis, and hypersensitivity reactions to sirolimus or stent polymers may have contributed to the serious major adverse cardiac events experienced in some patients treated with DES1-5,10-18. Daemen and his co-workers have also reported that late stent thrombosis was encountered steadily with no evidence of diminution in rates at up to three years of follow-up, and more recently, Wenaweser and his colleagues from the Bern and Rotterdam groups reported 4-year data suggesting that definite stent thrombosis occurs steadily at an annual rate of 0.4% to 0.6%13,14. The BASKET-late trial showed that the rates of late death or MI were more than three times higher in the DES arm than BMS arm after the discontinuation of clopidogrel (from seven to 18 months)17. These concerns highlight the need to continue to explore alternative strategies for reducing the burden of restenosis.
The cutting balloon is a unique device consisting of a balloon catheter with three blades that create longitudinal incisions in the atherosclerotic lesion during balloon inflation. In REDUCE III, we hypothesised that the use of IVUS guidance could be essential for obtaining optimal results with a strategy of cutting balloon angioplasty (CBA) prior to bare metal stenting, because IVUS facilitates optimal device sizing based on vessel size and plaque distribution, and can reveal vessel wall injury after CBA18. We performed a multicentre randomised study comparing 7-month clinical and angiographic outcomes in 521 patients with or without IVUS-guidance, as well as CBA or balloon angioplasty (BA) prior to BMS (REDUCE III)18.
All 521 patients were divided into four groups based on device and IVUS use before bare metal stenting (IVUS-CBA-BMS, Angio-CBA-BMS, IVUS-BA-BMS, and Angio-BA-BMS). While all four groups were well matched without significant differences in baseline clinical, demographic and angiographic characteristics, the restenosis rate we obtained (6.6%) was significantly lower in the IVUS-CBA-BMS group18. Based on these low restenosis rates, we further hypothesised that IVUS-guided CBA prior to BMS could, by facilitating full stent expansion with safety, improve accommodation of reactive intimal hyperplasia, and thereby produce favourable long-term restenosis rates comparable to those achieved with DES in similar circumstances.
To test this hypothesis, we compared 7-month clinical and quantitative coronary angiographic (QCA) outcomes between the IVUS-guided CBA-BMS strategy (derived from the randomised Restenosis Reduction by Cutting Balloon Angioplasty Evaluation study, REDUCE III) and a DES-based strategy (derived from the Rapamycin-Eluting-Stent Evaluation At Rotterdam Cardiology Hospital study, RESEARCH)19,20. Patients from each study were matched for previously reported principal predictors of restenosis – vessel size (RD-pre), lesion severity (MLD-pre), lesion location of the arteries and the presence of diabetes7,21-23.
Methods
IVUS-guided CBA and BMS group (from REDUCE III)
The design of the randomised Restenosis Reduction by Cutting Balloon Angioplasty Evaluation (REDUCE III) study has been previously reported18. In brief, all 521 patients were divided into four groups based on device and IVUS use before bare metal stenting (IVUS-CBA-BMS: 137 patients; Angio-CBA-BMS: 123; IVUS-BA-BMS: 142; and Angio-BA-BMS: 119). At follow-up, residual diameter stenosis was significantly less in the IVUS-CBA-BMS group than the others (p=0.016 by ANOVA). The restenosis rate in the IVUS-guided CBA with BMS group (6.6%) was significantly lower than the Angio-CBA-BMS (17.9%); IVUS-BA-BMS (19.8%); and Angio-BA-BMS groups (18.2%, p=0.016). Outcomes for the IVUS-CBA-BMS group were then compared to those obtained in similar patients who underwent drug-eluting stent implantation in the RESEARCH study.
All percutaneous coronary intervention (PCI) procedures were performed according to standard clinical practice via radial or femoral approaches using guide catheters 6 Fr or greater in size to facilitate subsequent QCA analysis. No drug eluting stents were used in the REDUCE III study. CBA, BA and BMS sizes were determined by angiographic reference vessel diameter in the angiography-guided group and by IVUS-detected vessel dimension and plaque distribution in the IVUS-guided group. The following IVUS criteria for optimal stenting originally derived from the MUSIC study were used: 1) good stent apposition with symmetrical stent expansion; 2) full stent expansion with sufficient lumen area (lumen area 80% or greater of the average reference lumen area pre-intervention); and 3) the absence of major dissection18,24. In REDUCE III, angiographic follow-up (206±75 days) was obtained in 453 patients out of 521 patients (87% of eligible patients).
DES from RESEARCH study
The design of the Rapamycin-Eluting Stent Evaluation At Rotterdam Cardiology Hospital (RESEARCH) study has been previously reported19,20. In brief, the use of a sirolimus-eluting stent (Cypher; Cordis, Johnson & Johnson, Warren, NJ, USA) was introduced as the default strategy for all patients undergoing PCI at the Erasmus Medical Center for six months. All procedures were performed according to standard techniques, and the final interventional strategy was left to the discretion of the operator, with the aim of achieving a final residual stenosis <50% by online quantitative coronary angiography in the presence of TIMI (Thrombolysis In Myocardial Infarction) 3 grade flow. While the use of periprocedural glycoprotein IIb/IIIa inhibitors and antithrombotic medications was left to the discretion of the attending team, clopidogrel was prescribed for at least three months19,20. In the RESEARCH study, angiographic follow-up (204±34 days) was obtained in 441 lesions of 238 patients (70% of eligible patients).
Quantitative coronary angiography (QCA; REDUCE III and RESEARCH)
For both studies, QCA analyses were performed using the same computer-based edge-detection coronary angiography analysis system (CAAS II, Pie Medical, Maastricht, The Netherlands)18-20,25,26. Coronary angiograms were obtained in multiple views matched after the intracoronary injection of nitrates. Interpolated reference vessel diameter (RD), minimal lumen diameter (MLD) and percentage diameter stenosis were obtained at baseline (pre-), post-stenting and at follow-up using the guiding catheter as a scaling device by the same QCA system18-20,25,26. Restenosis was defined as >50% diameter stenosis at follow-up. QCA analyses in the REDUCE III study were performed at an independent core laboratory at the Aichi Medical University and Fujita Health University,
Aichi, Japan, while QCA analyses in the RESEARCH study were done at the Thoraxcenter, Erasmus University, Rotterdam, The Netherlands18-20.
Matching process (REDUCE III and RESEARCH)
The process of matching for angiographic and clinical characteristics has been previously described in detail21,22. In brief, vessel size and the presence of diabetes are known to be strong predictors of restenosis after BMS as well as DES7,23. Therefore, lesions were individually matched according to reference vessel diameter (RD-pre) and minimal lumen diameter (MLD-pre) by QCA, the presence or absence of diabetes, and lesion location of the arteries. The principles of QCA matching are threefold: 1) The angiographic dimensions of matched lesions and reference vessels are assumed to be “identical”; 2) the reference diameter of the lesions (RD-pre) to be matched is selected within a range of ±0.2 mm (2SD of the reproducibility of the CAAS analysis system); and 3) the observed difference between the two “identical” lesions (MLD-pre) must be within a range of ±0.3 mm (= 3SD of the reproducibility of the CAAS analysis (0.1 mm=1SD)21,22.
Automated matching was performed using a custom-written database analysis algorithm produced by the computer department at the Fujita Health University. This identified 120 coronary artery lesions with serial QCA from 120 patients of the IVUS-guided CBA-BMS arm in REDUCE III who could be individually matched with 120 prospectively collected lesions treated with DES and angiographic follow-up derived from the RESEARCH study. In the remaining 17 out of 137 patients (IVUS-CBA-BMS arm in REDUCE III), 15 did not have angiographic follow-up (no serial QCA available) and a further two patients who did not have restenosis could not be matched because no identical patients were found from the RESEARCH study according to the pre-specified matching criteria.
Angiographic and clinical endpoints
The primary endpoint of this study was angiographic restenosis (defined as >50 percent diameter stenosis at follow-up by QCA). The principal clinical endpoint was a composite of major adverse cardiac events (MACE) including death, newly developed Q-wave and non-Q-wave acute myocardial infarction (AMI), and need for target vessel revascularisation (TVR) including target lesion revascularisation (TLR), and additionally subacute stent thrombosis (SAT; <30 days after the procedure) as well as late stent thrombosis (LST; >30 days after the procedure)18-20.
Statistical analysis
Data were analysed using the SAS statistical software package (SAS Institute, Cary, NC, USA). All continuous variables are expressed as mean±SD. Differences in categorical variables were assessed using the chi-squared test and Fisher’s exact test. The unpaired t-test was used to assess differences in continuous variables between two groups and ANOVA for four groups. Where a significant difference was detected by ANOVA, multiple comparison analysis was performed to disclose where the significance existed among the four values27. A two-tailed value of p<0.05 was considered significant.
Results
Clinical and angiographic characteristics in the matched comparison of REDUCE III and RESEARCH
The automated matching program identified 120 coronary artery lesions with serial QCA from 120 patients in the IVUS-guided CBA-BMS arm of REDUCE III which could be individually matched with 120 prospectively collected lesions treated with DES and angiographic follow-up derived from the RESEARCH study (Table 1).
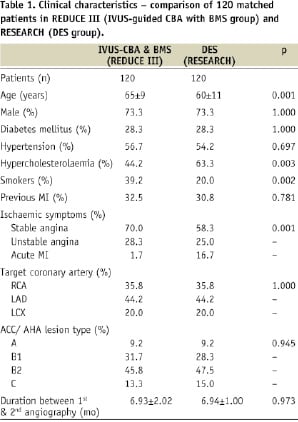
By this matching design, there was no significant difference in the incidence of diabetes, gender and lesion location of the arteries between the two groups. However, the average age was significantly greater in the IVUS-CBA-BMS (REDUCE III) group than that in DES (RESEARCH) group (Table 1). Although the incidence of hypercholesterolaemia was significantly higher in RESEARCH than in REDUCE III (p=0.003), the prevalence of smoking was higher in REDUCE III than in RESEARCH (p=0.002). While stable and unstable angina were more common in REDUCE III than in RESEARCH, acute myocardial infarction was more prevalent in RESEARCH (p=0.001). The ACC/AHA lesion types were similar for the two groups, as was the duration between PCI and follow-up angiography (Table 1).
Serial QCA, restenosis rate and target lesion revascularisation in the matched comparison of REDUCE III and RESEARCH
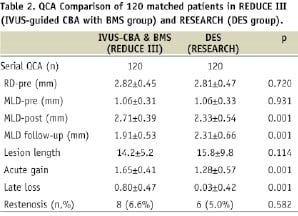
By the matching design employed, the vessel size (RD-pre), lesion severity (MLD-pre), lesion location of the arteries and the presence and absence of diabetes were identical in the 120 paired lesions from the IVUS-guided CBA and BMS group in REDUCE III and from DES group in RESEARCH. Lesion length was not statistically different between the two groups (Table 2).
Cumulative frequency curves illustrate the procedural and follow-up effects on MLD of IVUS-guided CBA with BMS (REDUCE III) and DES (RESEARCH) in the matched-paired lesions (Figure 1).
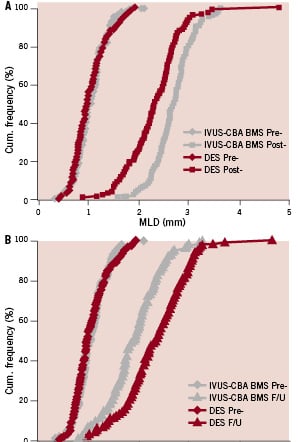
Figure 1. Cumulative frequency curves illustrating the procedural (1A, pre & post) and follow-up (1B, pre & follow-up) effects on MLD of IVUS-CBA-BMS and DES in the matched 120 paired lesions. The superimposed frequency distribution curves of MLD before PCI indicate similar preprocedural lesion severity. While MLD-post was greater in IVUS-CBA-BMS (REDUCE III) than in the DES group (RESEARCH) (2.71±0.39 mm vs. 2.33±0.54 mm, p=0.001) (Fig. 1A), MLD follow-up (F/U) was greater in DES than IVUS-CAB-BMS (2.31±0.66 mm vs. 1.91±0.53 mm, p=0.001) (Fig 1B).
The superimposed frequency distribution curves of MLD before IVUS-CBA-BMS (REDUCE III) and DES (RESEARCH) indicate similar pre-procedural stenosis severity (Figures 1A and 1B). While MLD-post was greater in the IVUS-guided CBA and BMS group (REDUCE III) than that in the DES group (RESEARCH) (2.71±0.39 mm vs. 2.33±0.54 mm, p=0.001), MLD follow-up was greater in the DES (RESEARCH) group than that in the IVUS-CBA-BMS (REDUCE III) group (2.31±0.66 mm vs. 1.91±0.53 mm, p=0.001) (Table 2 and Figure 1).
Whilst acute gain was significantly greater in the IVUS-guided CBA and BMS group (REDUCE III) than that in the DES group (RESEARCH) (1.65±0.41 mm vs. 1.28±0.57 mm, p=0.001), late loss was significantly less in the DES group than that in the IVUS-CBA-BMS group (0.80±0.47 mm vs. 0.03±0.42 mm, p=0.001) (Table 2). This inverted relationship of acute gain and late loss between IVUS-CBA-BMS and DES resulted in no statistically significant difference in restenosis rates (IVUS-CBA-BMS: 6.6% vs. DES: 5.0%, p=0.582) (Table 2).
Major adverse cardiac events (MACE)
Clinical follow-up was obtained in all the patients (100%) in both groups. All MACE are shown in Table 3.
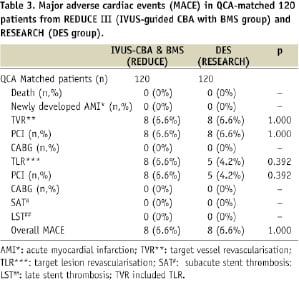
No deaths or newly developed AMI were observed in either group. While target lesion revascularisation (TLR) was done by PCI in eight (6.6%) patients in the IVUS-CBA-BMS group from REDUCE III, TLR was performed by PCI in five (4.2%) patients in RESEARCH (p=0.392). While TVR included TLR and revascularisation in different segments of PCI-treated segments in the same vessel, the TVR rates were subsequently similar (6.6% and 6.6%, respectively, p=1.000) between the QCA matched populations in both groups (Table 3).
Discussion
This QCA-matched comparison study has revealed that the greater acute gain achieved with IVUS-guided CBA and BMS (REDUCE III) and the smaller late loss attained with DES (RESEARCH) resulted in no significant difference in restenosis rates (IVUS-CBA-BMS; 6.6% vs. DES; 5.0%, p=0.582) and target vessel revascularisation rates (REDUCE III; 6.6% vs. RESEARCH; 6.6%, p=0.624). Subsequently, a similar MACE rate (REDUCE III; 6.6% vs. RESEARCH; 6.6%, p=0.624) was obtained between the two QCA matched groups.
“The bigger the better” strategy versus “the least late loss” strategy
While the acute gain achieved was significantly greater in the IVUS-guided CBA and BMS group (REDUCE III) relative to that obtained with the use of DES (RESEARCH) (1.65±0.41 mm vs. 1.28±0.57 mm, p=0.001), late loss was significantly less with DES than IVUS-CBA-BMS (0.80±0.47 mm vs. 0.03±0.42 mm, p=0.001). The two strategies, i.e., greater acute gain by IVUS-guided CBA and BMS – “the bigger the better” strategy – and negligible late loss by DES – “the least late loss” strategy, resulted in no statistically significant difference in restenosis rates (IVUS-CBA-BMS: 6.6% vs. DES: 5.0%, p=0.582) and target lesion revascularisation rates (REDUCE III: 6.6% vs. RESEARCH: 4.2%, p=0.392).
Necessity of long-term careful follow-up after DES
Although the RAVEL study indicated complete inhibition of restenosis, substantial growth in the real-life use of DES has revealed some potentially significant limitations, including an increased incidence of late and very stent thrombosis, leading to myocardial infarction or even death1-5,11-17,28-30. Recently, Bavry and his colleagues reported that both sirolimus and paclitaxel eluting stents significantly increased the risk of late thrombosis in comparison with BMS15. The BASKET-late trial showed that the incidence of late death or MI was more than three times higher in the DES arm than BMS arm of the study, after the discontinuation of clopidogrel (between seven and 18 months)17. Daemen and his co-workers have also suggested that late stent thrombosis was encountered steadily with no evidence of diminution up to three years of follow-up13. More recently, Wenaweser and his colleagues indicated that late stent thrombosis occurred steadily at an annual rate of 0.4% to 0.6% for up to four years from the Bern-Rotterdam database14. In our REDUCE study, late stent thrombosis did not occur in any patient with BMS.
Long-term safety data beyond five years has not yet been made available for DES in a large patient population, while conventional stent use has a history spanning two decades. Therefore, until long-term safety concerns have been allayed, there remains a need to explore alternative strategies for improving the outcomes achieved with conventional stent technologies and the use of IVUS-guided cutting balloon angioplasty with BMS is one such approach.
Study limitations
Firstly, matching of prospectively collected lesions is retrospective in nature and may have led to selection bias. For this reason, the matching technique is not superior to a prospective randomised study. Additionally, the matched DES arm was derived from an unrelated non-contemporaneous study, although there was some overlap period between the two studies. To minimise these differences, we performed the QCA measurements using an identical system and methodology.
Secondly, we matched strong restenosis predictors for bare metal and drug eluting stents, such as the presence of diabetes, vessel size and lesion severity, and found no difference in the lesion length or gender difference between IVUS-guided CBA and BMS from REDUCE III and DES patients from the RESEARCH registry. Nevertheless, it is possible that changes in practice over the course of the studies may have influenced results.
Finally, we were unable to construct a valid cost effectiveness model to compare IVUS-guided CBA and BMS carried out in Japan with DES performed in The Netherlands. International cost comparisons proved infeasible because of large differences in the cost of PCI devices in The Netherlands, Japan and the United States, and significant differences in medical reimbursement systems amongst these countries. While the initial device price of the IVUS catheter, cutting balloon and BMS seems to be lower than a single DES, the time required for the procedure is obviously longer in IVUS-CBA-BMS than simple DES.
Although it was difficult to derive universally applicable cost and time effectiveness models in this setting due to large international differences, drug-eluting stents are not suitable for all patients. A bare-metal stent based revascularisation strategy that confers low restenosis rates comparable to those obtained with drug-eluting stents may have benefits beyond cost, for instance, for those in whom prolonged dual antiplatelet therapy is undesirable either because of bleeding risk or need for elective surgery.
Conclusions
This QCA-matched comparison study between REDUCE III and RESEARCH has indicated that both the two strategies (i.e., greater acute gain by IVUS-guided CBA and BMS – “the bigger the better” strategy – and negligible late loss by DES – “the least late loss” strategy) resulted in similar restenosis rates between the two groups (p=0.582), suggesting that an IVUS-guided CBA prior to BMS strategy could be a viable substitute for DES in some clinical settings.
Acknowledgements
We would like to thank all the investigators of the REDUCE III study for their contributions, as well as all the staff of the Thoraxcenter, Erasmus Medical Center, Rotterdam, The Netherlands for carrying out the RESEARCH study.

