Transcatheter tricuspid valve replacement (TTVR) may provide a safe means of complete relief of tricuspid regurgitation (TR). Nevertheless, there are notable challenges, including the asymmetry and large size of the tricuspid annulus, potential interactions with the ventricular septum, and the short distance of the tricuspid annulus to the right ventricular free wall. Consequences of these concerns include conduction disturbances, ventricular pseudoaneurysms, excessive tricuspid and right ventricular enlargement, and reduced eligibility for TTVR therapy.
We herein describe a novel TTVR prosthesis (VDyne Valve; VDyne) whose design intent is to preserve the asymmetric shape of the tricuspid annulus and right ventricle. The VDyne system is composed of a double-frame, nitinol prosthesis, which houses a 30 mm porcine trileaflet valve (Figure 1). The outer frame is asymmetric, designed to fit a tricuspid annulus with a perimeter of up to 180 mm (i.e., 42 to 56 mm diameter). This asymmetry, with securement mechanisms at the right ventricular outflow tract (RVOT), ventricular free wall and posterior septum, permits minimal annular oversizing (i.e., <10%). The procedure does not require leaflet identification with echocardiography. To reduce the risk of ventricular afterload mismatch, a pop-off aperture can be employed to provide relief by allowing for a mild residual regurgitation. Standard anticoagulation with warfarin for >6 months is used after implantation to help minimise the risk of thrombogenicity.
A novel lateral approach towards the tricuspid valve and RVOT is used for deployment through a 28 Fr transfemoral system, with deflection capability for a range of inferior vena cava offset. The design allows the prosthesis to be crimped or folded vertically, rather than radially, leading to minimal interaction with the right ventricular apex and free wall that could be associated with risk of pseudoaneurysm. The distal aspect is passed over an extra-stiff guidewire placed into the left pulmonary artery to engage the RVOT. The proximal portion is unsheathed, and the ventricular aspect of the prosthesis is cinched sequentially in the anterior-posterior and septal-lateral dimensions with individual, ratcheted sutures. This cinching collapses the ventricular outer frame by ~25%, allowing the operator to advance the proximal portion over the posterior tricuspid annulus for deployment. Lateral guide deflection and steering facilitate perpendicular positioning to ease placement. The total implant height post-delivery along the lateral aspect of the frame is only 15 mm. The system does not require pacing for deployment. Importantly, it is fully retrievable after full expansion and positioning, permitting assessment of its effect and stability before final decoupling.
The first 3 patients with the fully retrievable VDyne prosthesis were all treated successfully as part of compassionate use therapy. The first patient was a 68-year-old woman with severe, New York Heart Association (NYHA) Class III symptoms of dyspnoea who had presented at Helsicore Hospital (Tbilisi, Georgia) with a predicted surgical mortality of 7.3%. She had torrential TR (effective orifice area 0.94 cm2). The right ventricle was mildly enlarged (4.9 cm) with mild dysfunction (tricuspid annular plane systolic excursion [TAPSE] 14 mm). Cardiac computed tomography (CT) imaging measured a tricuspid annular perimeter of 135 mm. A VDyne TTVR prosthesis was placed with 9% oversizing (Figure 1). At 6 months and thus far, she is asymptomatic. The second patient, who was referred to Sint-Jan Hospital (Bruges, Belgium), was a 58-year-old woman with NYHA Class III symptoms and torrential TR (effective orifice area 1.25 cm2) in the setting of 2 prior sternotomies for mitral surgery (predicted mortality 5.2%). On cardiac CT, the tricuspid annular perimeter measured 167 mm. Her coaptation gaps were large, and she was treated as part of a compassionate use program. A VDyne TTVR prosthesis was placed with 11% oversizing. A third patient, also treated at Helsicore hospital, was a 60-year-old woman with Class III symptoms and torrential TR, mild right ventricular (RV) dysfunction, an annular perimeter of 162 mm on CT, and a predicted mortality of 5.4%. For each of these 3 patients, a VDyne prosthesis was placed successfully, with elimination of TR and no major adverse events within 30 days. Repeat imaging for all 3 at 30 days showed normal prosthesis stability and function, preservation of the asymmetric shape of the RV, and elimination of TR. In all cases, RV function was stable.
These 3 cases demonstrate promising early feasibility. Future studies will determine the ultimate role of the VDyne TTVR system in the management of patients with severe TR.
In summary, TTVR with the VDyne prothesis offers a unique transcatheter solution for treatment of TR. The VDyne TTVR is a low-profile prosthesis that is delivered through a novel lateral approach in order to reduce risk of interaction with surrounding structures and utilises an asymmetric outer frame to minimise enlargement of the RV.
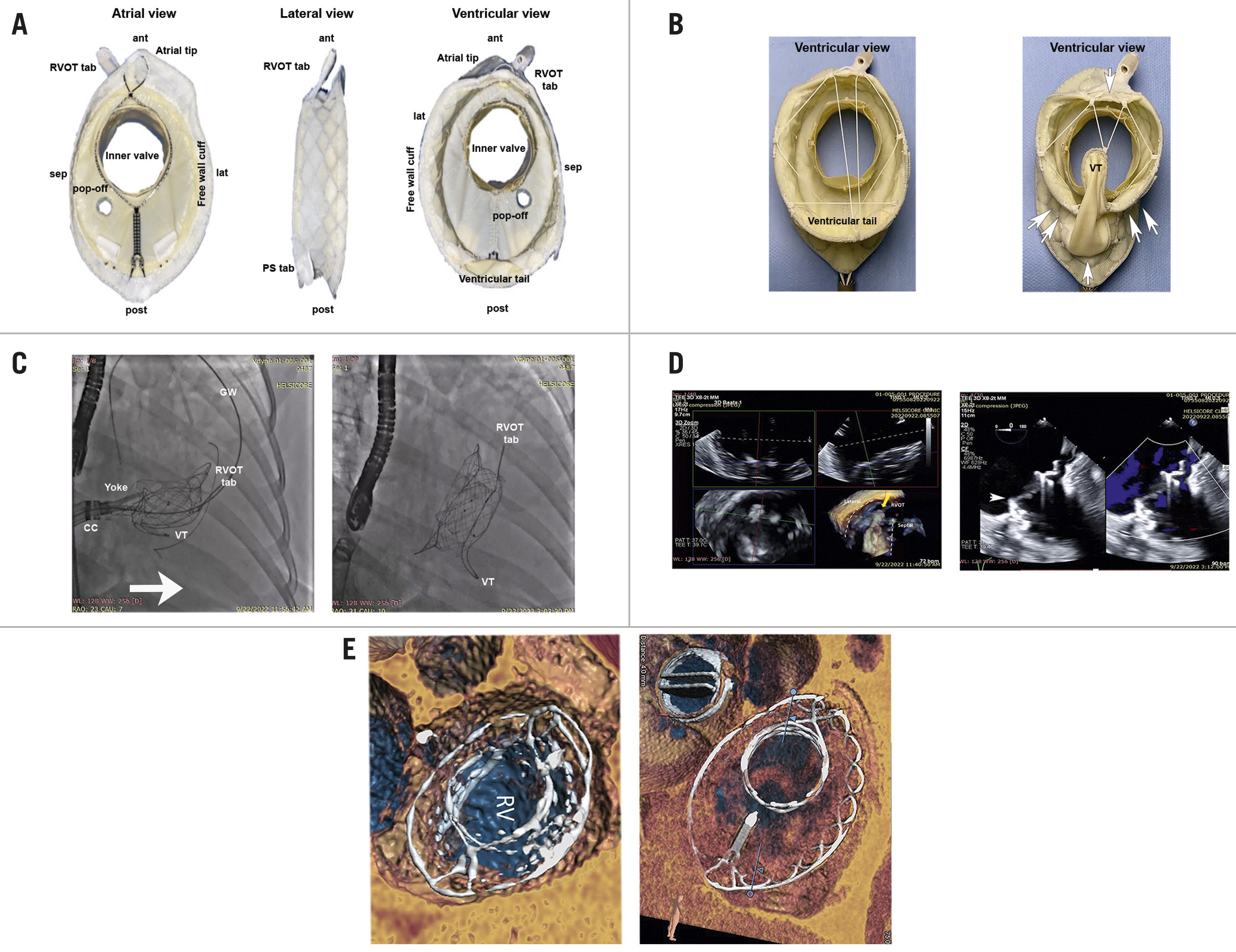
Figure 1. The VDyne transcatheter tricuspid valve replacement (TTVR) prosthesis. A) Detailed aspect views. B) Before (left) and after (right) cinching (arrows) reduces the ventricular profile of the prosthesis to enable seating. C) Left, superior passage along a guidewire passed through the right ventricular outflow tract (RVOT) enables seating using a low-profile approach (arrow) that is parallel to the tricuspid valve. Right, final seating. D) Left, transesophageal echocardiography guidance for placement of RVOT tab and superior aspect of the prosthesis. Right, colour-flow imaging shows elimination of tricuspid regurgitation by the VDyne prosthesis placement (arrowhead). E) Postprocedural CT images at 30 days for the first (left) and second (right) patients. ant: anterior; CC: control catheter; CT: computed tomography; GW: guidewire; lat: lateral; post: posterior; RV: right ventricle; sep: septal; VT: ventricular tail
Conflict of interest statement
P. Sorajja is a consultant for 4C Medical, Abbott Structural, Anteris, BioSense Webster, Boston Scientific, Edwards Lifesciences, Medtronic, Philips, Siemens, VDyne, W.L. Gore & Associates, and xDot. M. Burns is a consultant for VDyne. N. Hamid is aconsultant for Anteris, AMX, 4C Medical, Alleviant Medical, Edwards Lifesciences, Philips, GE HealthCare, Valcare Med Ltd, VDyne, and W.L. Gore& Associates. The other authors have no conflicts of interest to declare.

