Abstract
Aims: Percutaneous coronary interventions (PCI) evoke an inflammatory response and have been reported to decrease adiponectin levels. If persistent over time, the inflammatory response and low adiponectin levels would induce insulin resistance, rendering PCI potentially hazardous for glucose metabolism. We investigated whether PCI decreased insulin sensitivity after one month and if so, whether this was related to a lasting elevation of inflammatory markers and decrease in adiponectin levels.
Methods and results: Insulin sensitivity (euglycemic hyperinsulinaemic clamp) was measured in 19 patients with stable coronary artery disease before and one month after PCI. Also, levels of inflammatory mediators and adiponectin were assessed. Insulin sensitivity was not affected by the PCI procedure. There were no persistent increases in IL-6, hs-CRP and adiponectin levels after PCI.
Conclusions: The inflammatory response seen immediately after PCI does not translate to a medium-term negative effect on insulin action.
Introduction
Percutaneous coronary interventions (PCI) induce an inflammatory response early after the procedure, as reflected by an elevation of plasma high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6) levels.1 Recently, the acute inflammatory response evoked by PCI has been suggested to also reduce levels of adiponectin.2 As inflammation and low adiponectin levels are associated with insulin resistance, the PCI-induced inflammatory response itself might render patients insulin resistant in the early phase after PCI.3 Here we report the results of a study in which we assessed insulin sensitivity before and one month after PCI. In addition, levels of inflammatory parameters and adiponectin were measured.
Methods
Nineteen patients with stable angina referred for elective PCI were included (male/female: 12/7, aged mean ±SD 59±8 years, BMI 27.8±4.0 kg/m2, CCS class 2.9±0.6). Exclusion criteria were: reduced left ventricular ejection fraction (<40%); underlying infectious, inflammatory or malignant disease; diabetes; treatment with anti-inflammatory or immunosuppressive drugs.4 The institutional review committee approved the study and informed consent was obtained from all patients before participation in the study.
PCI was performed according to standard procedures. Insulin sensitivity was measured by a hyperinsulinemic (60 mU/m2/min) euglycemic clamp procedure.5 IL-6, hs-CRP and adiponectin were measured prior to the start of the clamp procedure in arterial blood samples. Samples were immediately centrifuged, and serum was collected in 2 ml microtubes and stored at –80 degrees Celsius until batch analysis. Adiponectin was measured using a Human Adiponectin (Acrp30) enzyme-linked immunosorbent assay (ELISA) Kit (DuoSet®; R&D Development Systems, Minneapolis, MN, USA), assay range 61.5-4000 pg/ml. High-sensitivity C-reactive protein (hsCRP) was measured by enzyme-immunoassay according to the instructions from the manufacturer (Dako, Glastrup, Denmark), assay range 0.049-25 ng/ml. Il-6 was measured by ELISA (PeliPair™ Reagent Set Sanquin; Sanquin, Amsterdam, The Netherlands), assay range 1.56-200 pg/ml. Measurements were performed the morning prior to PCI and one month after PCI. All experiments were conducted at 8:00 in the morning after an overnight (10-hour) fast with the patient in supine position in a quiet temperature-controlled room (23-24 °C). After complete instrumentation, 30 minutes of rest were included to attain stable baseline before data collection.
Statistical analysis
Data are expressed as mean±SEM unless indicated otherwise. Serum levels of IL-6, hs-CRP and adiponectin were not normally distributed and were summarised by medians and interquartile ranges. Differences between visits were assessed using parametric (student t-test for paired observations) or nonparametric (paired Wilcoxon) tests as appropriate. A two-tailed p-value of <0.05 was considered to be statistically significant. Correlations between parameters were calculated using Spearman correlation coefficients.
Results
All patients completed the study; PCI was successful in all patients. Most patients had single vessel disease, mean number of implanted stents 1.4±0.6. Medication was identical at both visits (aspirin n=19, beta-blocker n=18, statin n=16, ACEI n=4, calcium channel blockers n=5), except for five patients who used clopidogrel due to drug-eluting stent implantation at the second visit. No difference was found between the patients with our without clopidogrel. There was no significant difference in body weight before and one month after PCI.
Glucose levels during the clamps were stable (mean coefficients of variance: before: 4.2±0.6 and after PCI 3.7±0.4%). Four weeks after PCI, insulin sensitivity was similar to baseline: glucose infusion rate (GIR), reflecting whole-body glucose utilisation, was alike before and one month after PCI (Figure 1). Analogously, PCI had no effect on the insulin sensitivity index (43.9±5 vs. 45.3±5.1 mg/kg/ µU/min, p=NS).
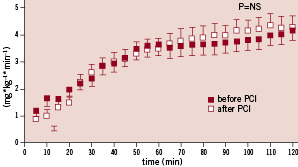
Figure 1. Glucose infusion rate (GIR) before and after PCI.
One month after PCI, IL-6 and hs-CRP levels were not persistently elevated, but returned to baseline values (median <3.0 before PCI vs. <3.0 pg/mL 1-month after PCI for IL-6, median 1.5 before PCI vs. 1.1 mg/L 1-month after PCI for hs-CRP). IL-6 and hs-CRP levels were well correlated (baseline, rho=0.48, p<0.05 and after PCI, rho=0.44, p=0.06). PCI had no medium-term effect on adiponectin levels (median 2.8 before PCI vs. 2.9 mg/mL 1-month after PCI). Hs-CRP and IL-6 levels did not correlate with adiponectin levels.
Discussion
Our data show that the increase in IL-6 and hs-CRP seen immediately (<48h) after PCI does not persist one month after PCI. Also, adiponectin levels were similar one month after PCI. Insulin sensitivity was not affected by the PCI procedure. This is in accordance with the absence in change in inflammatory markers and adiponectin levels.
When compared to published values using an identical set-up, glucose infusion rates during the clamp in this group were clearly below normal.6 Thus, as a group, they were obviously insulin resistant, which is consistent with the situation of chronic myocardial ischaemia.
Our study was not designed to determine immediate changes in inflammatory markers after PCI, nor can statements be made regarding cause-effect relationships between the different parameters as only correlations were assessed. Although relatively small-sized, the clear equality of our data should make different results from a larger cohort, highly unlikely. It was calculated that the study had enough statistical power to rule out any potential clinically relevant changes in hs-CRP and IL-6. As the inflammatory response (<48-72h) immediately following PCI has been studied extensively, it was the focus of the present study to assess medium-term (one month) effects of PCI on insulin resistance. We did confirm the short-term inflammatory response to PCI in our own institution (data not shown). Irrespective of the change in inflammatory markers or underlying mechanism, our study provides evidence that PCI has no effect on insulin sensitivity one month after the procedure. Suggested PCI hazards on insulin sensitivity via the inflammatory or adiponectin pathway seem therefore highly unlikely.
In conclusion, the inflammatory response seen immediately after PCI in CAD patients, does not persist for one month after PCI. In accordance, plasma adiponectin levels and insulin sensitivity remain unaffected. Thus, the inflammatory response seen immediately after PCI does not translate to a medium-term negative effect on insulin action.

