
Abstract
Aims: The aim of this study was to evaluate the relationship between body mass index (BMI) and outcomes in patients with coronary artery disease undergoing percutaneous revascularisation.
Methods and results: In 13 randomised trials, 22,922 patients were stratified (in kg/m2) as underweight (BMI <18.5), normal weight (18.5 ≤BMI <25, used as reference), overweight (25 ≤BMI <30), and obese (Class I [30 ≤BMI <35], Class II [35 ≤BMI <40], or Class III [BMI ≥40]). The primary endpoint was all-cause death at five years. Secondary endpoints were cardiac and non-cardiac death, target (TLR) and non-target lesion revascularisation (NTLR), myocardial infarction (MI), and definite/probable stent thrombosis. Despite adjustment for multiple confounders, overweight and Class I obesity were associated with lower all-cause mortality versus normal weight (HR 0.83, 95% CI: 0.71-0.96, and HR 0.83, 95% CI: 0.69-0.96, respectively); however, non-cardiac death was the major contributor to this effect (HR 0.77, 95% CI: 0.63-0.94 for overweight). Conversely, cardiac mortality was higher in severely obese individuals (HR 1.62, 95% CI: 1.05-2.51 for Class III obesity). Obesity was associated with higher rates of NTLR (HR 1.28, 95% CI: 1.04-1.58 for Class II obesity) but not with TLR, MI and stent thrombosis.
Conclusions: Moderately increased BMI is associated with improved survival post PCI, mostly due to lower non-cardiac but not cardiac mortality.
Introduction
It is estimated that high body mass index (BMI) contributes to 4.0 million deaths per year worldwide and the prevalence of obesity continues to increase1. Obesity and excess body fat are strong risk factors of premature cardiovascular and non-cardiovascular diseases; yet, in patients with known coronary artery disease (CAD) and in those undergoing percutaneous coronary intervention (PCI), being overweight is associated with a significant all-cause mortality benefit2,3. Despite previous reports describing the so-called “obesity paradox”, there is still a need for more data on the exact causes and durability of the observed mortality benefit and its association with certain clinical and procedural characteristics, especially the extent of the cardiac and non-cardiac mortality effect and the relationship between body size and other cardiovascular outcomes. Thus, we investigated five-year cardiovascular outcomes in relation to baseline BMI using pooled patient-level data from randomised controlled trials (RCTs).
Methods
STUDY POPULATION
Individual patient-level data from 21 PCI RCTs performed and reported between 1999 and 2016 were pooled in a centralised database at the Cardiovascular Research Foundation (New York, NY, USA). For the present analysis, 13 studies with available BMI at the time of PCI were included (Supplementary Table 1). All but three trials (ACUITY, HORIZONS-AMI, SPIRIT IV) reported five-year outcomes. The trials compared different types of stent (bare metal stents, first- and second-generation drug-eluting stents [DES]) and various antithrombotic regimens (heparin with or without glycoprotein IIb/IIIa inhibitors, or bivalirudin) in patients with a spectrum of clinical presentations. All studies complied with the Declaration of Helsinki and were approved by the institutional review boards at each participating centre; written informed consent was obtained from all patients at the time of enrolment.
Patients were stratified into six groups using the National Heart, Lung, and Blood Institute definitions according to their BMI (kg/m2) at the time of PCI: underweight (BMI <18.5), normal weight (18.5 ≤BMI <25), overweight (25 ≤BMI <30), obesity Class I (30 ≤BMI <35), obesity Class II (35 ≤BMI <40), and obesity Class III (BMI ≥40)4.
STUDY ENDPOINTS
The primary outcome of the study was all-cause death at five-year follow-up. Secondary outcomes included cardiac and non-cardiac death, ischaemia-driven target lesion revascularisation (TLR), non-target lesion revascularisation (NTLR) in the target vessel and non-target vessel, myocardial infarction (MI), and definite/probable stent thrombosis as defined by the Academic Research Consortium5. All MIs within three days post PCI were classified as periprocedural and were excluded because of the variable definitions used. Definitions of cardiac and non-cardiac death were similar across studies, with deaths of unknown cause considered cardiac. All outcomes were reported in all studies included in the analysis, except for NTLR which was not available in ACUITY, TAXUS II, IV, V and PLATINUM.
STATISTICAL ANALYSIS
Patient-level data were pooled as one structured data set. Baseline demographics, core lab reported quantitative coronary angiography results, and procedural characteristics are presented as mean and standard deviation and were tested with the Student’s t-test (after normal distribution was confirmed with the Kolmogorov-Smirnov test). Multiple group comparisons were performed using analysis of variance. Categorical variables are presented as counts and percentages and were tested with the χ2 test. Because we expected less than one false positive test on average if all BMI pairs tested positive at a 5% type I error, no adjustment of p-values for post hoc pairwise testing was done. Cumulative five-year event rates are presented as Kaplan-Meier estimates and were tested with the log-rank test. A multivariable Cox proportional hazards model was adjusted for patient and lesion characteristics with each RCT as a random effect using normal weight individuals as a reference. Selection of covariates was based on their historical and pathophysiologic association with risk of ischaemic events and differences across BMI subgroups. The following covariates were included in the model: age, sex, diabetes mellitus, current smoker, hypertension, hyperlipidaemia, previous coronary artery bypass grafting, previous PCI, previous MI, clinical presentation with MI (non-ST-elevation myocardial infarction [NSTEMI] or ST-elevation myocardial infarction [STEMI]) versus stable CAD or unstable angina, stent type (bare metal stents, first-generation DES, second-generation DES), stent length, reference vessel diameter, calcification, and number of treated lesions. All study outcomes were tested for interaction with age, sex, clinical presentation, and stent type. Two-sided p-values <0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Overall, 22,922 patients (mean age 62.8 years, 27.8% female) were included. BMI was 29.0±5.4 kg/m2. When divided into BMI subgroups, 137 (0.5%) were classified as underweight, 4,997 (21.8%) as normal weight, 9,665 (42.2%) as overweight, and 8,123 (35.4%) as obese, including 5,318 (23.2%) Class I, 1,903 (8.3%) Class II, and 902 (3.9%) Class III. Baseline patient clinical characteristics are summarised in Table 1. Overweight and obese patients were younger than those of normal weight. Higher BMI was associated with more cardiovascular risk factors (diabetes mellitus, hypertension, and hyperlipidaemia) as well as higher rates of prior MI and prior revascularisation (both PCI and surgery), while current smoking had a lower prevalence across higher BMI subgroups. Higher BMI was associated with more frequent stable CAD than normal BMI and underweight individuals.
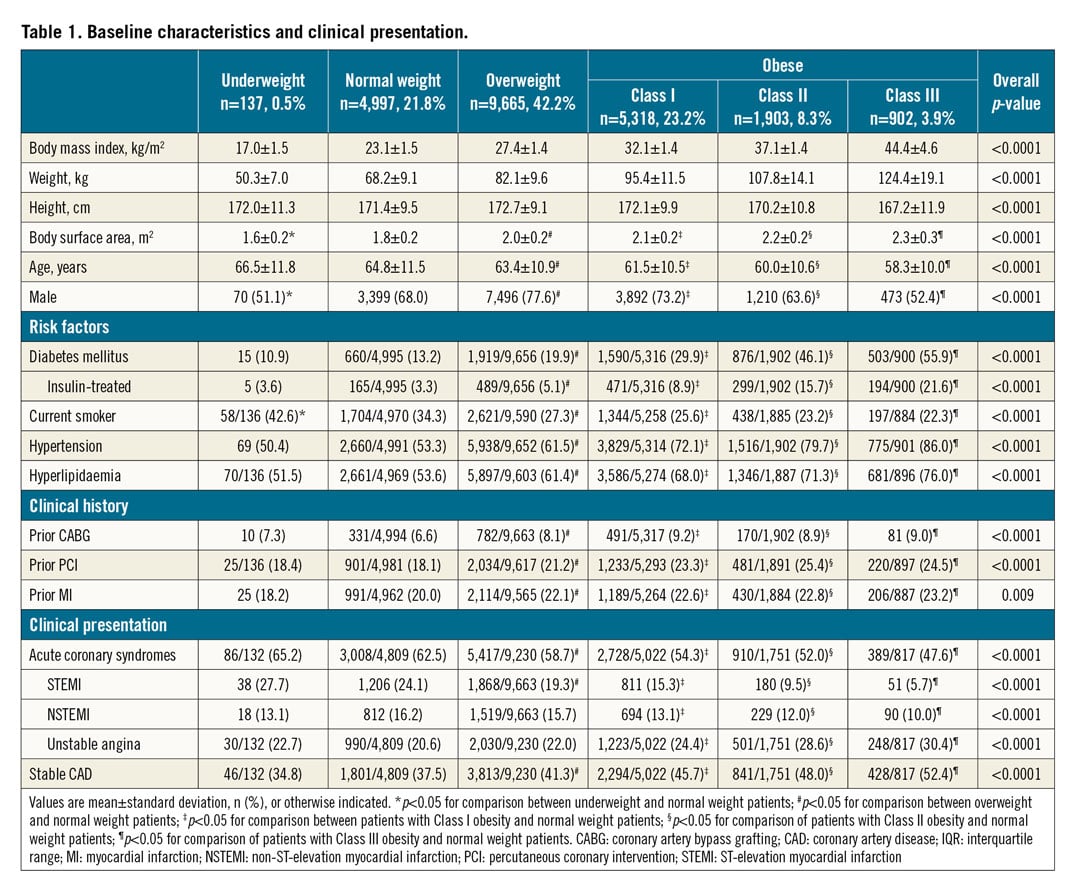
Core lab angiographic findings and procedural characteristics are presented in Table 2. Overall, 80% of patients underwent single-vessel PCI (1.2±0.6 lesions), and 86% of stents were DES (58.5% second-generation). Reference vessel size and minimal lumen diameter were larger in higher BMI patients versus normal weight counterparts. In overweight and obese patients, lesions were shorter, less calcified, and less frequently ACC/AHA type C versus normal weight patients. PCI procedures in obese, but not overweight patients were associated with significantly shorter stents, but greater residual diameter stenosis versus normal weight individuals.
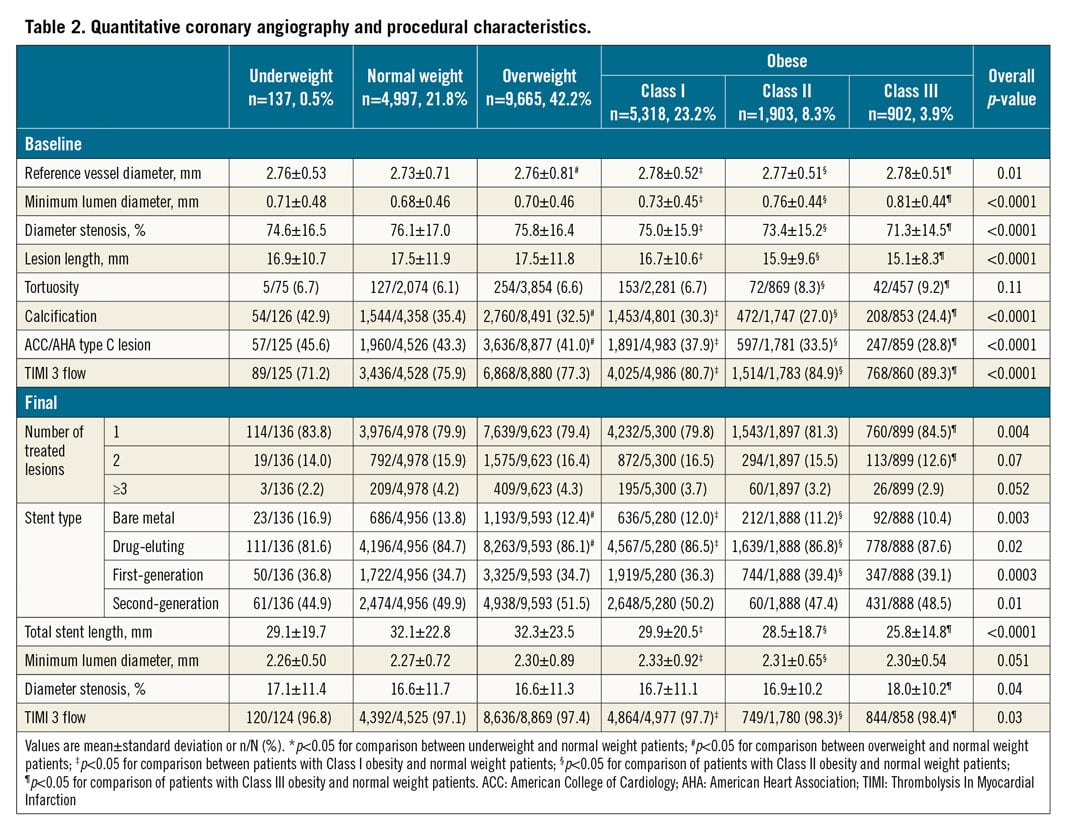
At five-year follow-up, 1,450 (9.2%) deaths were reported (683 [47.1%] cardiac and 767 [52.9%] non-cardiac) along with 1,762 (9.4%) ischaemia-driven TLR, 1,543 (14.6%) NTLR, 876 (4.8%) MIs, and 385 (2.1%) definite/probable stent thrombosis (Supplementary Table 2).
Kaplan-Meier curves for outcomes across BMI subgroups are presented in Figure 1 with unadjusted and adjusted hazard ratios (HR) summarised in Figure 2, Figure 3 and Supplementary Table 3. Spline curves representing the association of BMI with unadjusted and adjusted risk of study outcomes are shown in Supplementary Figure 1 and Supplementary Figure 2. Unadjusted all-cause mortality was twofold higher in underweight (HR 2.15, 95% confidence interval [CI]: 1.38-3.33) and was lower in overweight (HR 0.75, 95% CI: 0.66-0.85), obese Class I (HR 0.67, 95% CI: 0.57-0.78), and obese Class II patients (HR 0.70, 95% CI: 0.57-0.87) versus normal weight individuals. After adjustment, overweight and Class I obesity remained related with lower all-cause mortality (HR 0.83, 95% CI: 0.71-0.96, and HR 0.83, 95% CI: 0.69-0.99, respectively).
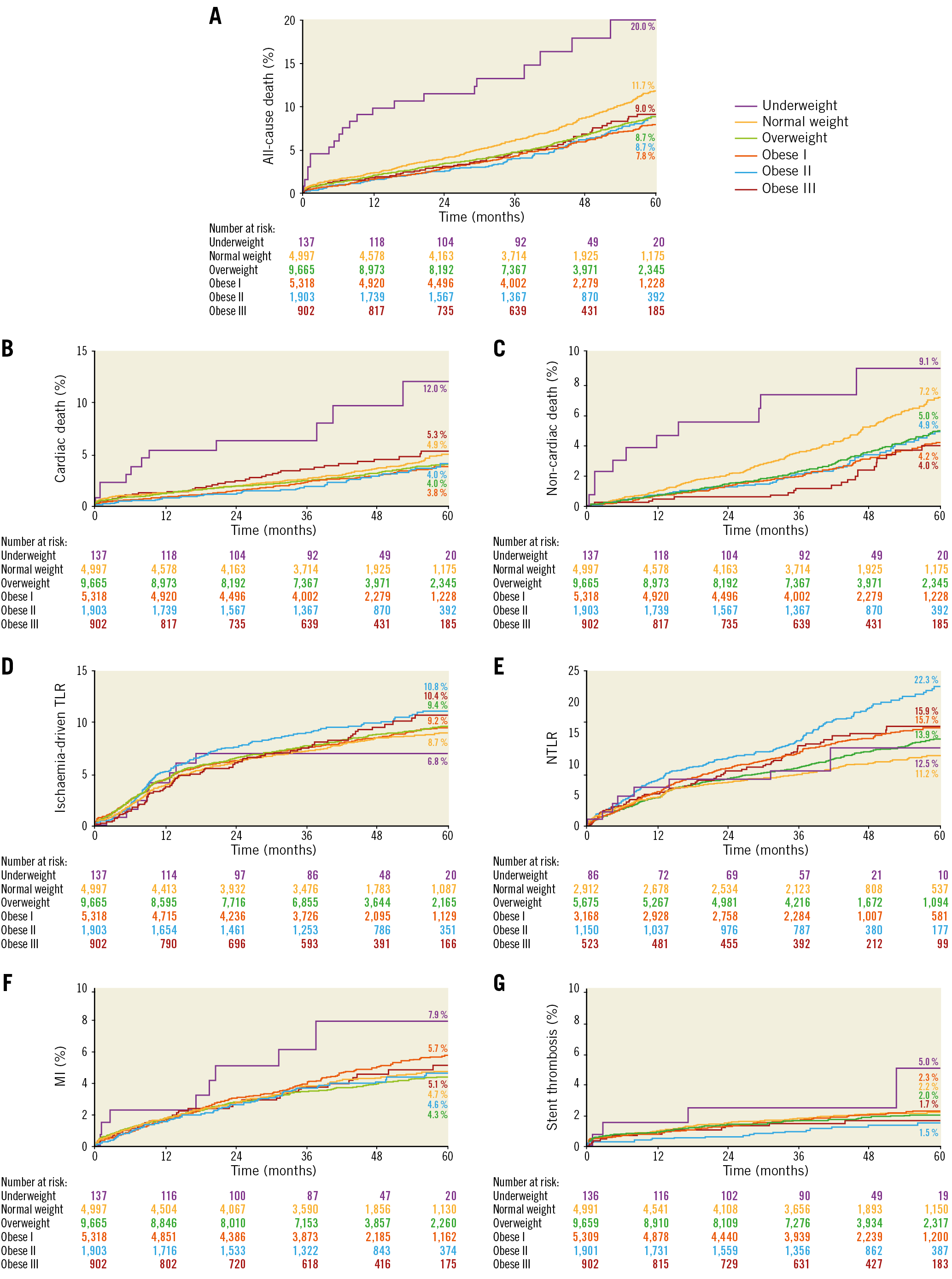
Figure 1. Kaplan-Meier curves stratified by body mass index.
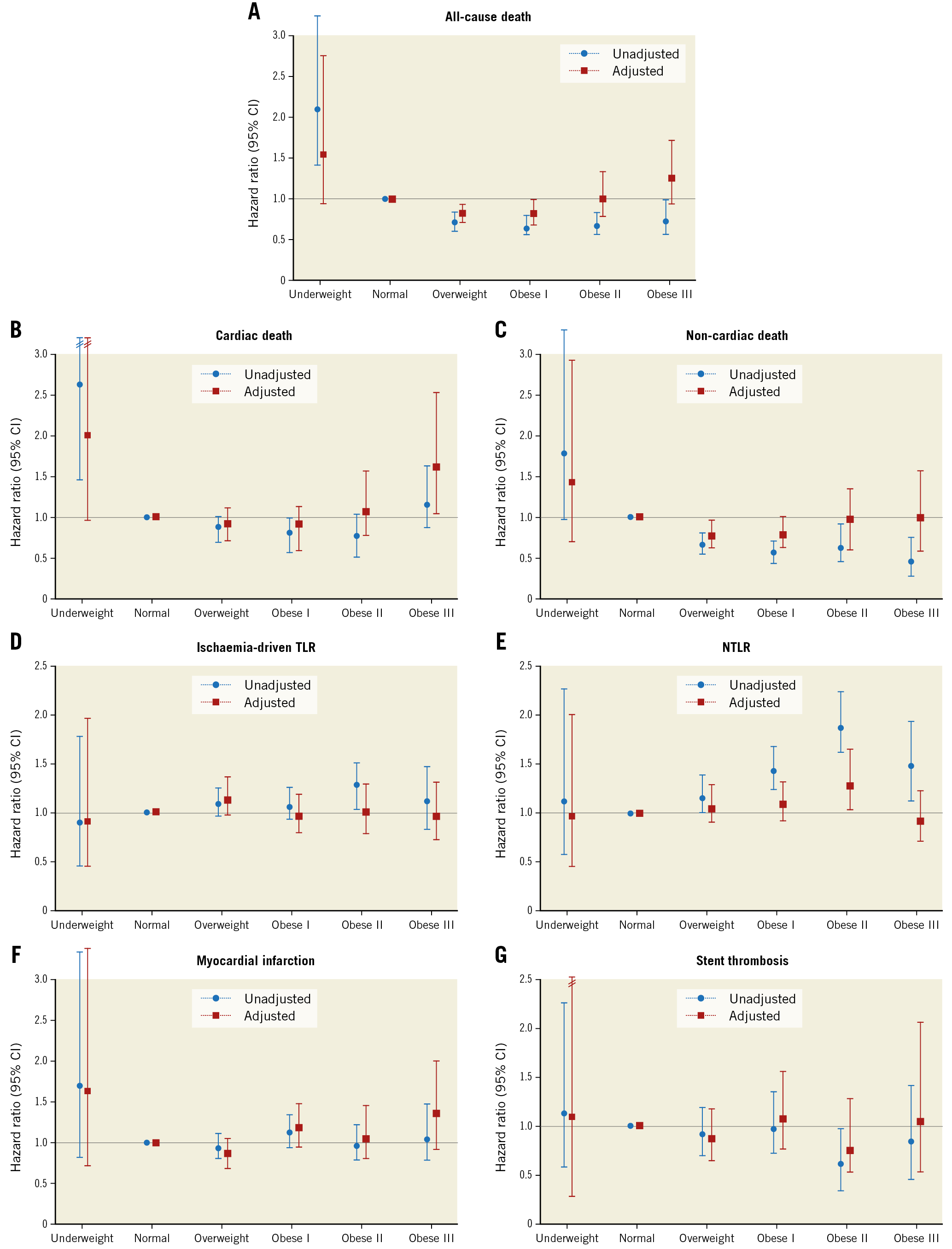
Figure 2. Unadjusted and adjusted risk of study outcomes according to body mass index. Normal weight was used as a reference. CI: confidence interval
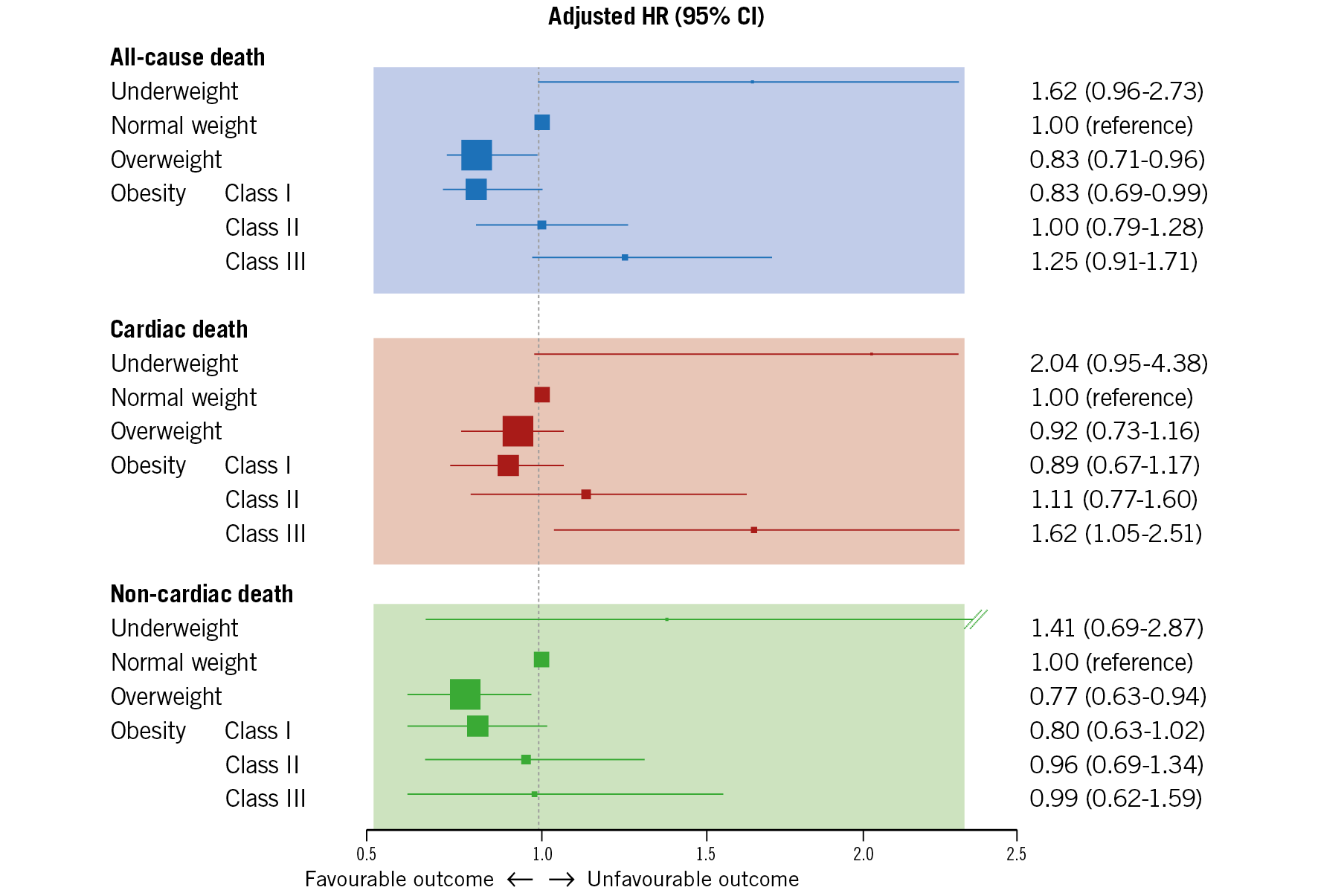
Figure 3. Adjusted all-cause, cardiac and non-cardiac mortality five years after percutaneous coronary intervention. Box sizes represent number of patients in each BMI subgroup. HR: hazard ratio
Rates of cardiac death were higher in underweight (HR 2.65, 95% CI: 1.44-4.89) and lower in obese Class I patients (HR 0.79, 95% CI: 0.63-0.99) versus normal weight individuals. After multivariable adjustment, excess body weight was associated with higher cardiac mortality, statistically most significant for Class III obesity (HR 1.62, 95% CI: 1.05-2.51).
Unadjusted non-cardiac death was markedly lower in overweight (HR 0.67, 95% CI: 0.56-0.80) and obese Class I to III patients (HR 0.57, 0.65, and 0.48, respectively) versus normal weight patients. The trend persisted after adjustment, and overweight remained associated with improved non-cardiac mortality (HR 0.77, 95% CI: 0.63-0.94).
There were no differences in unadjusted and adjusted rates of ischaemia-driven TLR across BMI subgroups; however, rates of NTLR were higher for overweight (HR 1.16, 95% CI: 1.01-1.35) and Class I to III obesity subgroups (HR 1.41, 1.85, and 1.47, respectively). After adjustment, obesity Class II was an independent predictor of NTLR (HR 1.28, 95% CI: 1.04-1.58). No differences were found in rates of MI across BMI categories. The unadjusted risk of definite/probable stent thrombosis was lower in the Class II obesity versus normal weight subgroups (HR 0.60, 95% CI: 0.37-0.96). After adjustment, none of the BMI subgroups was associated with a risk of stent thrombosis. The unadjusted and adjusted adverse event rates were similar when only studies with five-year follow-up were included (Supplementary Table 4, Supplementary Table 5, Supplementary Figure 3).
Subgroup analyses of patients treated with second-generation DES and other stents were performed (Supplementary Table 6, Supplementary Table 7). We found no significant interaction between the use of second-generation DES and adjusted study outcomes at five years, except for ischaemia-driven TLR (pinteraction=0.04) (Supplementary Table 8). Similarly, no significant interaction regarding study outcomes was found with age (<65 vs ≥65 years), sex, BMS (vs DES) and clinical presentation (STEMI and NSTEMI vs stable and unstable angina) with the exception of stent thrombosis that was more prevalent in patients with biomarker-positive presentation (pinteraction<0.001) (Supplementary Table 8, Supplementary Table 9). Comparison of clinical and procedural characteristics, as well as unadjusted five-year outcomes of patients with and without available BMI data, are shown in Supplementary Table 10-Supplementary Table 12.
Discussion
The main findings of this large study using pooled patient-level data from 13 RCTs evaluating long-term outcomes after PCI are as follows. (i) Despite adjustment for multiple clinical, angiographic, and procedural confounders, BMI-determined overweight and moderate obesity were associated with a significantly lower long-term all-cause and non-cardiac mortality versus normal weight patients undergoing PCI. (ii) Conversely, cardiac death was not reduced in overweight and moderately obese patients but was significantly higher in morbidly obese individuals. (iii) A high BMI was associated with an increased risk of NTLR, but not with rates of ischaemia-driven repeat target lesion PCI, spontaneous MI, or stent thrombosis. (iv) Underweight patients comprised a small fraction of PCI subjects; they had the highest long-term mortality.
The advantage of the present study over previously published reports is in providing data not only on all-cause mortality, but also on centrally adjudicated cardiac and non-cardiac causes of death. Moreover, the large amount of clinical and procedural information available for patients enrolled in the analysed RCTs allowed adequate statistical adjustment. In the largest single study to date (N=345,192), Holroyd et al6 reported that overweight and obesity (BMI >30 kg/m2) were independent predictors of lower all-cause five-year mortality. However, the study did not distinguish between cardiac and non-cardiac death, similar to the study by Hastie et al7 which reported significantly lower adjusted five-year all-cause mortality of overweight, but not obese patients undergoing elective PCI. All-cause and cardiac mortality were evaluated in a pooled patient-level analysis of women undergoing PCI in 26 RCTs reported by Faggioni et al8, revealing the association of higher BMI with lower all-cause, but not cardiac mortality.
Likewise, we reported a significant overall (cardiac and non-cardiac) mortality benefit to being overweight or obese. However, unlike these studies we revealed that only non-cardiac mortality was significantly lower; we found higher cardiac mortality in the highest BMI (>40 kg/m2) versus normal weight individuals. This finding confirms the results of a large meta-analysis (N=250,152) showing that severe obesity (BMI >35 kg/m2) was an independent predictor of higher cardiovascular mortality versus normal weight (relative risk 1.88, 95% CI: 1.05-3.34)2. Thus, the obesity paradox applies only to non-cardiac mortality, but not to cardiac mortality post PCI.
Aside from providing clarification on mortality patterns, our analysis revealed an association between BMI and higher rates of NTLR, a surrogate of CAD progression. This explanation seems plausible, given the higher prevalence of comorbidities and cardiovascular risk factors in obese patients as well as the previously reported association between obesity and accelerated development of CAD9. Conversely, the reduced anatomical complexity of CAD in obese patients was probably due to younger age of presentation versus normal weight counterparts. Closer medical attention and potentially a lower threshold for follow-up in secondary prevention of obese patients may also contribute to this effect10.
In the current analysis there was no association between BMI and long-term risk of ischaemic TLR. Several previous trials assessing outcomes post PCI reported significant associations between higher BMI and increased risk of TLR and target vessel revascularisation11,12; however, the studies comprised mostly data on patients treated with bare metal stents. In studies reporting outcomes post DES, a high BMI was not associated with an increased risk of TLR or target vessel revascularisation13,14. One meta-analysis combining 15 studies (N=49,002) revealed a linear relationship between a higher BMI and any repeat revascularisation without categorisation into TLR and NTLR; however, again this was no longer significant when only patients treated with DES were included15. In the only previous study that distinguished TLR from NTLR, Wang et al16 analysed outcomes of 6,083 patients (median 26-month follow-up) undergoing elective PCI with DES and reported a significant association of a higher BMI with higher risk of NTLR, but not TLR, concordant with our results. Moreover, fewer calcifications, shorter lesion length, and larger reference vessel size in high BMI patients may contribute to good acute and long-term stent results.
The higher long-term mortality observed in our study in underweight versus normal weight individuals was largely consistent with previous reports2,6,7,8. The underweight population was characterised by the most advanced age, highest proportion of women, current smokers, and unstable clinical presentation, and the most coronary calcification. These are known predictors of worse outcomes following PCI. We hypothesise that underweight patients bear either advanced cardiovascular disease (e.g., chronic insulin-dependent diabetes) or some non-cardiovascular comorbidities affecting nutrition status (i.e., illness-related weight loss) despite the fact that patients diagnosed with cancer or other severe chronic illnesses were not included in most stent trials. Low BMI is also associated with greater response to antiplatelet and anticoagulant pharmacotherapy, leading to increased risk of bleeding events, which might have contributed to higher mortality10,17.
The absence of significant interaction between second-generation DES and study outcomes indicated that the observed relationships of BMI and all-cause, cardiac, and non-cardiac mortality as well as NTLR can be applied to contemporary clinical practice, largely dominated by newer DES platforms. The only significant interaction of stent type was with ischaemia-driven TLR. However, it seemed to be driven mainly by differences in the BMI <18.5 group (Supplementary Table 4, Supplementary Table 5), the group with the smallest sample size, thus possibly occurring by chance.
Finally, hazard ratios of all-cause, cardiac, and non-cardiac death across BMI subgroups form U-shaped curves (Figure 2), with the lowest number of events corresponding to overweight patients (25 ≤BMI <30 kg/m2). Many potential reasons for this apparent survival benefit have been suggested. The first is purely mechanistic and includes statistical explanations such as unmeasured confounders, reverse causation, and collider stratification bias, all described in detail elsewhere18. Despite selecting multiple covariates with potential impact on ischaemic outcomes for the multivariate Cox regression model, it is likely that some were not accounted for. However, as the adjustment revealed significant reduction of the point estimates observed in the unadjusted data, we hypothesise that most causes of improved survival in overweight patients are favourable differences in comorbidities, clinical presentation, and procedural factors. Second is the inherent limitation of BMI as a measure of obesity, as it does not differentiate whether the excess weight comes from fat or muscle. Other anatomical measures such as waist circumference or waist-to-hip ratio might give a better estimation of abdominal obesity because they have been shown to predict adverse outcomes independently of BMI; however, to date there are no studies assessing long-term prognosis of patients with CAD in relation to those indices. Third, the overweight subgroup was by far the largest (42.2%), twice the size of the normal weight reference (21.8%); therefore, it may be argued that this represents the “new normal”.
Study limitations
First, the studied cohort included only patients enrolled in RCTs who usually represent a lower-risk population. Second, no data on renal function, non-cardiac comorbidities, medications, PCI access site, or bleeding outcomes were available in our pooled data set. Third, possible changes in BMI at follow-up were not accounted for. Fourth, MI definitions varied among the studies, not surprising considering the time course in which these studies were conducted. Some of the associations might have occurred by chance due to multiple testing. Finally, we used BMI as a measure of obesity, reflecting body size, but could not assess its composition, including the amount of fatty tissue.
Conclusions
Our results from a large pooled patient-level analysis confirm the long-term survival benefit seen in overweight and moderately obese patients versus normal weight controls post PCI. The main contributor to the overall survival benefit was non-cardiac death; we found no protective effect of high BMI on cardiac death. Patients with high BMI represented a higher risk of NTLR, but not TLR.
|
Impact on daily practice Interventional cardiologists should no longer consider obese patients with stable CAD being at lower risk of adverse cardiovascular outcomes versus normal weight individuals. Even though patients with moderately increased BMI have improved long-term all-cause mortality post PCI, this results mostly from lower non-cardiac mortality as there was no significant association of BMI and the risk of MI, TLR, and stent thrombosis. Conversely, patients with severe obesity were at higher risk of both cardiac death and NTLR. Obese patients undergoing PCI should therefore receive adequate information on their risk of cardiovascular events and professional counselling about safe and effective ways to lose weight. |
Guest Editor
This paper was guest edited by Adnan Kastrati, MD; Deutsches Herzzentrum, Munich, Germany.
Funding
This investigator-sponsored study was funded by Abbott.
Conflict of interest statement
A. Maehara reports research grants and personal fees from Boston Scientific and Abbott Vascular. C. von Birgelen reports institutional research grants from Abbott Vascular, Biotronik, Boston Scientific and Medtronic, outside the submitted work. P.W. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, Qualimed, and Xeltis, outside the submitted work. R. Mehran reports grants from Abbott Vascular, Boston Scientific, Medtronic, AstraZeneca, Bayer, BMS, CSL, DSI, Novartis, OrbusNeich, Osprey Medical, and PLC/Renal Guard, and personal fees from Abbott Vascular, Medtronic, Medscape/Web MD, Siemens Medical Solutions, Philips/Volcano/Spectranetics, Roviant Sciences, Sanofi (ITA), Bracco Group, Beth Israel Deaconess, Janssen, Watermark Research, Medintelligence (Janssen), ACC, Bayer, and AMA, outside the submitted work. G. Stone reports personal fees from Terumo, Amaranth, Shockwave, Valfix, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, Sirtex, Ancora, Qool Therapeutics, SpectraWave, and MAIA Pharmaceuticals, and other from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, MedFocus family of funds, SpectraWave, and Orchestra Biomed, outside the submitted work. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

