
The clinical benefit of coronary artery stent implantation is largely determined by its ability to suspend reversible ischaemia, while the risk of thrombotic stent occlusion as well as haemorrhagic and vascular complications arising from concomitant pharmacological therapy counterbalance this clinical benefit. The introduction of dual antiplatelet therapy (DAPT) after stenting was a major breakthrough in the history of interventional cardiology1. Coronary stent placement requires a minimum of one month of DAPT (a combination of acetylsalicylic acid [ASA] and a P2Y12 inhibitor) after bare metal stent (BMS) implantation and six months of DAPT after placement of a drug-eluting stent (DES) per ACCF/AHA/SCAI 2016 and 2017 ESC/EACTS guidelines on myocardial revascularisation and duration of DAPT in patients with coronary artery disease (class I recommendation, level of evidence A)2. Although modified recommendations for patients at high bleeding risk (HBR) have been included in most recent guidelines, data to support these recommendations remain scarce and the level of evidence is low.
Preclinical studies have historically been undertaken to examine the biocompatibility of novel stent platforms in a comparative manner, in which recovery of the endothelial monolayer after stenting was demonstrated to play a crucial role in mitigating the risks of stent thrombosis3.
In the current issue of EuroIntervention, Jinnouchi et al compare the Polyzene-F® fluoropolymer nanocoated COBRA PzF BMS (CeloNova BioSciences, San Antonio, TX, USA) to contemporary DES in different preclinical settings with respect to endothelial recovery and permeability as well as inflammation and resistance against thrombus formation4.
To examine acute thrombogenicity, the COBRA PzF stent, durable (dp) and biodegradable (bd) polymer DES were implanted into silicone tubes in an experimental porcine arteriovenous shunt model showing a significantly lower number of thrombi (>0.1 cm2) by scanning electron microscopy (SEM) and markers of platelet adhesion by confocal microscopy (CM) in COBRA PzF and dp DES versus bd DES. Although such experimental models cannot fully mimic the complexity of human coronary artery disease, the observed low thrombus formation on the COBRA PzF stent surfaces may be an indicator of low thrombogenicity – an important surrogate of acute stent thrombosis.
Endothelial coverage was evaluated in an in vivo rabbit model after 14 days, showing significantly greater endothelial coverage in COBRA PzF stents as compared to all DES by SEM and by CD31 positive area using CM. Although not specific for endothelial cells, increased CD31 expression implies earlier endothelial recovery in COBRA PzF stents as compared to their drug-eluting comparators, probably due to the presence of very thin struts and the absence of antiproliferative drug in this surface-modified BMS. Rapid endothelial regrowth is an important hallmark of vascular healing5 as it represents recovery of the innate protective mechanism against platelet adhesion and activation of blood-borne coagulation factors6. Furthermore, it prevents cell invasion of the vessel wall and seals the underlying prothrombotic tissue shortly after stent-induced vascular injury6. Stent struts lacking endothelialisation have been shown to be an important characteristic of late and very late stent thrombosis, especially in first- and second-generation DES7.
Endothelial permeability at 28 days after implantation was assessed by quantification of p120/vascular endothelial cadherin complex using CM, which co-localises in areas with intact and functional endothelial cell borders and was significantly greater in COBRA PzF as compared to all DES. Evans blue dye uptake, a marker of incomplete endothelial barrier function8, was least in the COBRA PzF when compared to dp and bd DES, indicating reduced endothelial permeability in the COBRA PzF stent. Precise regulation of endothelial permeability is essential to prevent uncontrolled invasion of blood-borne leucocytes and lipoproteins into the vessel wall through leaky endothelium. Therefore, regeneration of endothelial integrity after stenting plays a critical role in the prevention of atherosclerotic lesions within stented vessel segments (in-stent neoatherosclerosis) and probably also for subsequent thrombotic events9.
Jinnouchi et al further evaluated vascular inflammation by quantification of neutrophil (PM-1) and monocyte markers (CD14) using CM in the arteriovenous shunt model for acute adhesion of inflammatory cells and by quantification of RAM-11 positive area representing macrophage infiltration in the 14-day chronic stent implantation model. Immunostaining for both PM-1 and CD14 showed significantly lower inflammatory cell adhesion on both the COBRA PzF and dp DES as compared to the bd-coated DES. Conversely, the macrophage positive area was significantly lower when compared to its comparators, as evaluated by immunostaining against RAM-11. Neutrophils are critical for early formation of prothrombotic neutrophil extracellular traps and have been identified as a hallmark of stent thrombosis10, while monocytes and macrophages are known to play a major role in the pathogenesis of in-stent neoatherosclerosis9. Therefore, low adhesion of inflammatory cells on stent surfaces might mitigate early vascular inflammation and prevent untoward thrombotic events.
The COBRA PzF BMS enables rapid endothelial regrowth and low thrombogenicity, which are important requirements to shorten concomitant DAPT from a preclinical perspective. In this regard, it promises a compromise position for HBR patients in need of short DAPT duration and yet reliable antirestenotic efficacy. Preclinical studies such as the current one provide important insights into clinically translatable endpoints when compared to contemporary DES. Unfortunately, the authors of this study omitted to inform us about the comparative antirestenotic efficacy of the COBRA PzF coronary BMS relative to DES in their study. This would have been helpful for a comprehensive understanding of its clinical utility and broader vascular performance. When compared to other BMS, the COBRA PzF stent met performance goals derived from a meta-analysis of historical bare metal stent trials as assessed in the COBRA PzF SHIELD trial11. However, no data are available for the assessment of restenosis rates in comparison to contemporary DES.
Clinical implications
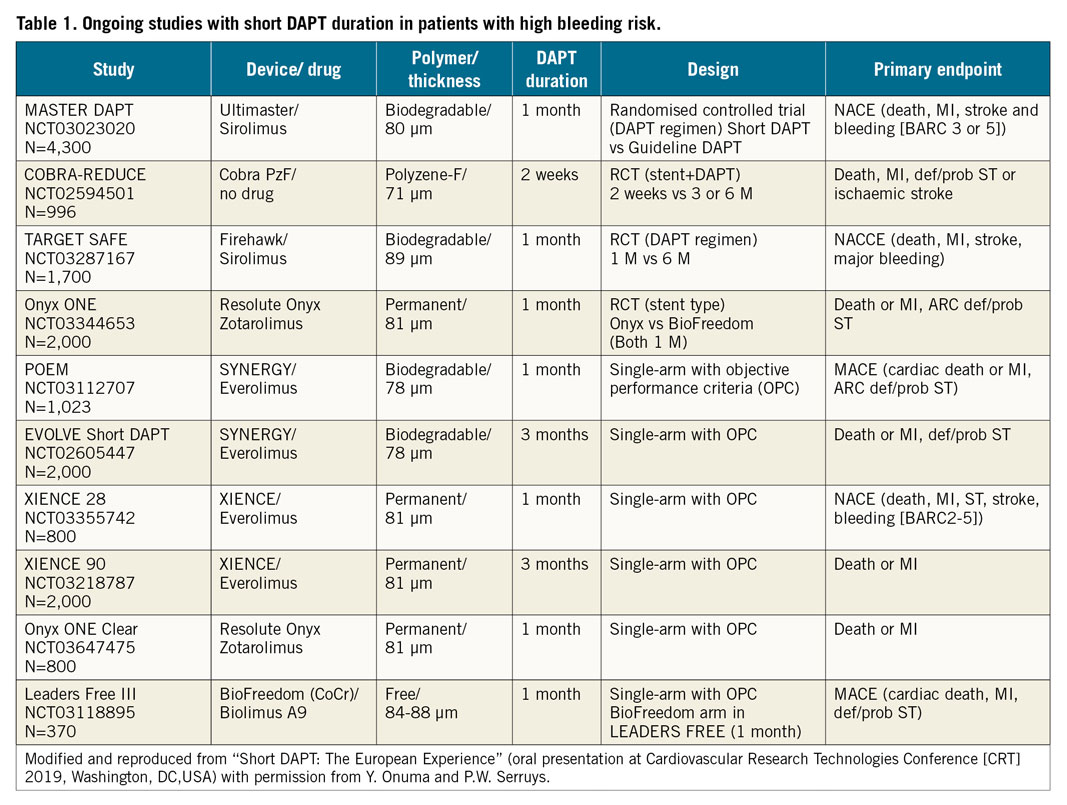
HBR patients, such as those treated with oral anticoagulation (OAC) therapy, are at additional risk when undergoing percutaneous coronary intervention (PCI) and were either excluded or underrepresented in most clinical trials designed to test the efficacy and safety of DES. The accepted practice in such patients was BMS implantation with one month of DAPT, eventually accepting higher rates of restenosis when compared to DES. Until recently, even more inclusive studies of contemporary DES continued to exclude patients for whom guideline-recommended DAPT was considered unsuitable12. Therefore, combining OAC with DAPT, resulting in triple antithrombotic therapy after PCI, needs to be intensively scrutinised along with alternative stent designs. Currently, a number of randomised clinical trials investigating short DAPT durations are ongoing in PCI patients perceived to be at increased bleeding risk (Table 1). The wealth of trials applied to study truncated DAPT after DES placement demonstrates the gap in knowledge encountered in daily practice but is also based on the commercial interest of stent manufacturers trying to propagate market shares amongst a growing population in need of urgent medical attention. We observe with concern that the majority of these trials are based on rather a weak justification to shorten DAPT, in the absence of specifically designed preclinical studies to investigate relevant endpoints of vascular healing, such as was performed in the current study. HBR patients might benefit from novel stent designs such as the COBRA PzF, which was shown to reduce thrombogenicity and accelerate endothelial healing relative to contemporary DES. However, the question remains whether the absence of drug release from this stent will turn into increased restenosis rates and whether such an adverse outcome may translate into inferior clinical performance. A randomised clinical trial using the COBRA PzF stent is ongoing in HBR patients with only two weeks of DAPT followed by a single antiplatelet regimen relative to Federal Drug Administration (FDA)-approved DES with standard DAPT duration to confirm its safety and efficacy (COBRA-REDUCE trial; NCT02594501). This might provide further insights into how short DAPT duration can be safely limited in these high-risk patients.
In summary, the findings of Jinnouchi et al suggest a favourable safety profile of the COBRA PzF stent in comparison to contemporary DES, exhibiting promising characteristics for use in HBR patients where a shortened DAPT duration is needed. Whether passive polymeric stent coatings such as that applied on this stent hold promise and balance safety with efficacy will be answered in dedicated clinical trials.
Conflict of interest statement
T. Koppara has no conflicts of interest to declare. M. Joner reports receiving funding for the COBRA Reduce trial at the German Heart Center Munich.

