Abstract
A series of interventional tools have emerged since the advent of percutaneous coronary angioplasty. Several are fundamental and used routinely, while others less favourable have fallen short of mainstream therapy and/or have settled as a niche device. We present an overview of the evolution of directional coronary atherectomy (DCA), a unique device that was originally conceived in 1984 to solve the limitations of balloon angioplasty. Unfortunately, we have witnessed its use fall significantly out of favour due to premature and controversial study results. In many interventional laboratories DCA is no longer available. However, we strongly feel that allowing DCA to join the list of extinct interventional tools would be very unfortunate. We, herein, present a series of complex percutaneous coronary procedures to illustrate the convenience of DCA use as a lesion-specific niche device. Finally, DCA offers a valuable distinct clinical research function as it allows for in vivo pathological coronary tissue examination. In conclusion, we plead for its continued production and use as an interventional niche device for the wellbeing of our patients.
Introduction
The use of directional coronary atherectomy (DCA) has dramatically diminished since the advent of coronary stents. In many interventional laboratories, this device is no longer available. However, we feel that allowing DCA to join the list of extinct interventional tools would be unfortunate. DCA (alone or as adjunctive treatment before coronary stenting) may be the best treatment modality in selected cases such as origin LAD lesions and some bifurcations, warranting its preservation as a niche device in the coronary interventional armamentarium. In this article, we provide a thorough overview of DCA, including its evolution in clinical practice, the controversial landmark trials, and a few illustrative cases deemed worthy for its applicability.
Historical perspective
The concept of DCA was originally developed by John Simpson in 1984, when he invented a catheter-mediated technique to remove atherosclerotic plaque from coronary arteries. By removing obstructive plaque, he sought to reduce the high (30-50%) rates of restenosis observed with balloon angioplasty.1-4 Mechanically, the AtheroCath™ (Abbott Vascular, Redwood City, CA, USA) consists of a cylindrical metal housing with a lateral side window cutter and a low pressure balloon on the contralateral side. Low pressure balloon inflations allow for proper tissue-cutter apposition. The plaque is gently “pushed” into the window housing and “shaved-off” into the collecting nose cone at the distal tip of the device. Since plaque excision results in larger lumen diameter and less vessel wall trauma than balloon angioplasty, it was proposed that DCA would result in lower restenosis when compared to angioplasty “bigger is better”5,6.
From 1986 to 1989, DCA was utilised in approximately 1,020 procedures (1,140 lesions) among 14 clinical centres2,3. In an early multi-centre registry, DCA was successful in 85% of cases, and was thus subsequently comparable to balloon angioplasty2. Consequently, DCA was FDA approved for use in September 1990 as a first alternative to balloon angioplasty. By 1992, the use of coronary atherectomy devices increased rapidly and accounted for approximately 10% of non-surgical coronary revascularisation procedures in the United States7.
However, by the mid 1990s, conflicting results from randomised control trials questioned the clinical utility and applicability of DCA. While this was interpreted as a device limitation, other potential reasons included intention-to-treat study designs in which variable operator experience and skills resulted in less than complete tissue removal8,9. Moreover, with the advent of coronary stents, the clinical utility of DCA was further questioned given the excellent results and greater ease of use of stents. On the other hand, proponents of DCA have long believed that this technique may be successfully performed in selected patients with great angiographic results, particularly when employed by skilled operators proficient in “optimal” debulking.
DCA clinical trials
We review here the landmark trials that have impacted on the evolution and clinical utility of DCA, from the initial experience of the unfavourable CAVEAT to the concept of debulking and “optimal” DCA.
Initial experience – “minimalist” DCA
The Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT I) was the first multi-centre international randomised control trial (35 sites in the United States and Europe), comparing DCA to balloon angioplasty3. In this study, 1,012 patients were randomly assigned to either DCA (n=512) or angioplasty (n=500) with the primary endpoint of restenosis by angiography at six months. Unfortunately, this early DCA trial demonstrated no clinical benefit over balloon angioplasty in 6-month follow-up restenosis, with higher rates of death or myocardial infarction than balloon angioplasty (8.6% vs. 4.6%, p=0.007). DCA was also associated with higher rates of early complications (11% vs. 5%, p<0.001) and increased in-hospital cost ($11,904 vs. $10,637, p=0.006) as compared to angioplasty3. Despite the untoward clinical results, DCA did lead to an acutely larger luminal diameter (1.05 vs. 0.86 mm, p<0.001), more frequent luminal diameter reduction of less than 50% (89% vs. 80%; p<0.001), and trended towards lower follow-up restenosis rates (50% vs. 57% p=0.06) compared to balloon angioplasty. Of particular importance, DCA was superior to angioplasty for the treatment of bifurcating lesions. Similar results were reported with the use of DCA in saphenous vein graft interventions when compared to angioplasty10.
Further experience - “optimal” DCA
In the wake of the disappointing results from CAVEAT I, many felt that the failure of the study’s result was related to sub-optimal performance of this technique, with under-sized devices and little plaque removal. As Dr. Antonio Colombo said, “DCA does not work by ‘intention-to-treat’,” but only when the operator uses the device to achieve significant plaque removal and optimal lumen expansion, i.e., “optimal” atherectomy. “Optimal” DCA was later defined as an angiographic residual stenosis of than ≤15%8. Consequently, several “optimal” DCA clinical trials were conducted in cardiovascular centres with substantial DCA experience with encouraging results comparable to bare metal stenting (Table 1)8,11.
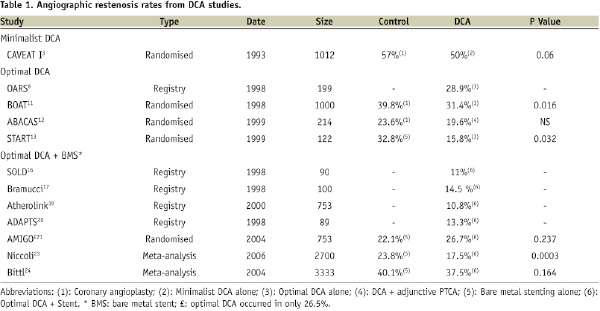
The Optimal Atherectomy Restenosis Study (OARS) was a prospective multicentre registry of 199 patients who underwent DCA for de novo or restenotic lesion8. Follow-up coronary angiography at six months was available in 83% of patients. Optimal DCA resulted in acute success in 97.5% of patients with an average residual stenosis of 7% of reference diameter, and complication rate of only 2.5%. Meaningful restenosis occurred in 28.9% of patients, which was lower than the previously reported 50% in CAVEAT3. In this study, acute luminal gain was identified as a major predictor of restenosis.
DCA versus PTCA
Balloon versus Optimal Atherectomy Trial (BOAT) was a multi-centre randomised control trial examining “optimal” DCA vs. angioplasty in 1,000 patients with single, de novo, native vessel coronary disease11. In this trial, the investigators conclude that “optimal” DCA provides greater short-term success, lower residual stenosis, and significantly improved long-term angiographic restenosis over angioplasty. “Optimal” DCA resulted in successful revascularisation with less than 15% luminal diameter when compared to 28% in the angioplasty arm. Long-term angiographic assessment at a mean of 7.2 months was available in 80% of patients and showed significantly lower angiographic restenosis rates in favour of “optimal” DCA when compared to balloon angioplasty (31.4% versus 39.8%; P=0.016). Overall mortality, target-vessel failure, and target-site and vessel revascularisation were similar at 1-year follow-up11.
DCA plus PTCA
In the Adjunctive Balloon Angioplasty After Coronary Atherectomy Study (ABACAS) the investigators sought to determine the short- and long- term benefits of balloon angioplasty adjunctive to DCA versus DCA alone12. In this trial, 214 patients who underwent intravascular ultrasound (IVUS) guided DCA with angiographically optimal debulking were randomised to either: a) no further treatment or b) additional angioplasty. Adjunctive PTCA significantly increased acute angiographic luminal gain and decreased acute angiographic restenosis than DCA alone, however it did not significantly impact the clinical and angiographic outcome at 6-month follow-up12.
DCA versus stenting
The Stent Versus Directional Coronary Atherectomy Randomised Trial (START) was a small trial comparing primary bare metal stenting to “optimal” DCA13. One hundred and twenty-two lesions were randomly assigned to either Palmaz-Schatz stenting (62 lesions) or DCA (60 lesions). Single or multiple stents were implanted with high-pressure dilation, while IVUS-guided debulking was performed in the DCA arm. Both invasive angiography and IVUS were performed pre- and post-procedure, and at six months follow-up. Immediate post-procedural lumen diameters were similar in both groups (2.79 vs. 2.90 mm, stent vs. DCA), however significantly smaller during follow-up angiography in the stent group (1.89 vs. 2.18 mm; p = 0.023). The degree of intimal proliferation by IVUS was significantly greater in the stent group than in the DCA group (3.1 vs. 1.1 mm; p < 0.0001), which explained the smaller lumen area during follow-up in the stent arm (5.3 vs. 7.0 mm2; p = 0.030). Restenosis was defined as >50% luminal diameter and was significantly lower (32.8% vs. 15.8%; p = 0.032) in the DCA group, as was the trend for target vessel failure during 1-year follow-up (33.9% vs. 18.3%; p = 0.056)13.
DCA plus bare metal stenting
It has been observed that the degree of plaque burden and calcification interferes with optimal stent deployment and results in higher rates of in-stent restenosis14. This has led to the concept of “debulking”; a mechanical strategy used to decrease plaque burden, optimise stent expansion and improve final minimal lumen diameters (MLD). Studies have demonstrated that the neointimal hyperplasia of in-stent restenosis occurs greatest at the site of the underlying plaque14,15.
Therefore several theoretical advantages of debulking have been proposed and include: 1) reduction in plaque burden to maximise acute lumen gain with less vessel stretching; 2) lower need for high pressure balloon inflations resulting in deep arterial wall injury; 3) reduction in stent edge problems; 4) minimising plaque redistribution during coronary stenting (“snowplough effect”) and thus decreasing PCI-related side-branch occlusions and infarctions; 5) and possibly decreases restenosis although this remains controversial.
The debulking strategy using DCA prior to coronary stenting was initially examined in several registries. In the Stenting After Optimal Lesion Debulking (SOLD) registry, DCA reduced neointimal hyperplasia and the incidence of in-stent restenosis. None of the study patients in this registry experienced stent thrombosis, and restenosis occurred in only 11% of patients, of which 7% underwent target lesion revascularisation at an average of 18 months16. Bramucci et al reported their experience with debulking when compared to a matched control group of stenting alone. Debulking resulted in larger acute gains, and significantly lower late lumen loss, restenosis rate, and clinical events17,18. The Atherolink registry also found a reduction in restenosis using the synergistic approach of DCA and stenting19. In the Acute Directional Coronary Atherectomy Prior to Stenting (ADAPTS) registry, this approach was safe and yielded low angiographic restenosis rate (13.3%) in high risk patients with complex lesions20. These favourable findings resulted in a follow-up randomised control trial with DCA prior to stenting versus stenting alone (AMIGO trial)21.
In the AMIGO study, 753 patients with de novo or restenotic coronary lesions were randomly assigned to either DCA prior to stenting or stenting alone, and were followed post-procedurally for 12 months. The investigators found no significant differences in clinical outcomes and binary restenosis between the two groups (DCA + stenting 26.7% vs. stent alone 22.1%; p=0.237)21. However, like CAVEAT, “optimal” DCA was not consistently performed and debulking standards were achieved in only 26.5% of patients in the debulking group.
Another study by Kim et al investigated the role of DCA plus stenting versus stenting alone in a specific subset of patients with ostial LAD lesions22. DCA plus stenting resulted in greater post-procedural luminal gain then stenting alone (4.0±0.4mm vs. 3.5±0.5mm, p<0.001), but did not improve long-term rates of angiographic restenosis (28.1% vs. 36.7% respectively, p=0.472). Nonetheless, a tendency towards lower restenosis and larger luminal gain was observed during follow-up in the DCA group, suggesting the possibility of a type β error. Furthermore, according to study’s IVUS analysis it is also possible that the negative results may have been explained by sub-optimal debulking.
Nevertheless, a meta-analysis by Niccoli et al involving 12 trials comparing DCA-debulking prior to stenting versus stenting alone showed debulking was superior and resulted in improved acute angiographic results and target lesion revascularisation with no difference in major adverse events. This analysis included a total of 1,216 patients with DCA-debulking prior to stenting and 1,484 patients with stent alone strategy. Debulking yielded greater acute luminal gain when compared to stenting, and was associated with significantly lower rates of angiographic restenosis (odds ratio [OR] 0.67, 95% confidence interval [CI] 0.54-0.84, p=0.0003) and target lesion revascularisation (OR 0.73, 95% CI 0.59-0.91, p=0.006)23.
In a more recent meta-analysis of five major DCA trials (AMIGO, BOAT, CAVEAT-I, CAVEAT II, and CCAT) involving 3,333 patients the authors showed a encouraging trend toward reduced restenosis with use of DCA (OR 0.9; 95% CI 0.77-1.05; p=0.164)24. Nevertheless, the trend was associated with an increased risk of periprocedural non-Q wave myocardial infarction at 30 days (OR 1.85; 95% CI 1.35-2.55).
DCA in the era of drug-eluting stents
The uprise of drug-eluting stents (DES) has dramatically reduced the rates of restenosis. Consequently, the use of coronary atherectomy catheters has continued to fall out of favour. Accordingly, the data examining the role of DCA in the current era of DES is scarce, and non-conclusive. Only a few studies are currently available and although encouraging, they are limited by their design.
According to the PERFECT registry25, debulking atherectomy prior to DES implantation in coronary bifurcation lesions results in lower rates of main branch (1.1%) and side-branch (3.4%) restenosis during 9-month follow-up angiography, and was associated with no deaths or non-fatal myocardial infarctions at one year. These findings are motivating, particularly when compared to the data of bifurcation stenting using DES alone reporting restenosis rates of up to 25%26-28.
In another study by Tanaka et al, plaque debulking prior to DES implantation in 101 patients with unprotected left main coronary artery bifurcation lesions resulted in significantly reduced restenosis rates at nine months when compared to the non-debulking strategy, particularly among the subset with ostial left circumflex disease (percent diameter stenosis 20.8±12.3% vs. 31.9±21.4%; p=0.007)29. We hope some of these findings will prompt the undertaking of new randomised control trials to further evaluate and understand the role of DCA in the era of DES.
We have learned that proper debulking preceding DES implantation is important. Not only does it facilitate stent delivery, but further allows for optimal stent expansion and adequate stent-to-arterial wall apposition. It is provocative to hypothesise that the use of atherectomy prior to DES implantation may in fact result in improved immediate outcome, lower restenosis and decreased incidence of late thrombosis during long-term follow-up. As proposed by Dr. Antonio Colombo, the use of DCA and DES implantation represents yet another logical combination waiting for evidence30.
Technical advances in DCA therapy
Flexi-cut technology
Technical aspects inherent to the DCA cutter have favourably evolved. The currently available lower profile systems have made the use of this device technically less challenging. Originally, the bulky DCA catheters required use of 11 Fr systems, and were particularly difficult to steer and manoeuvre. The development of the currently available 8 Fr Flexi-cut (Abbott Vascular, Redwood City, CA, USA) DCA catheter system with increased calibre and a more refined cutter offers further promise for improved DCA outcome. Takagi and colleagues31 studied 143 consecutive coronary lesions treated with Flexi-cut catheters as compared with 277 consecutive coronary lesions treated with the conventional 11 Fr system. The use of Flexi-cut resulted in larger post-procedural luminal diameter, greater luminal gain, a smaller number of DCA cuts, and a smaller number of residual stenosis (residual stenosis diameter of < 20% in 77% versus 45% patients). Additionally, O’Brien et al demonstrated the efficacy of DCA with Flexi-cut technology in patients with coronary in-stent restenosis32.
The SilverHawk (Fox Hollow Technologies, Inc.,Redwood City,CA, USA) cutter represents a novel plaque excision system not based on occlusive balloon inflation, but rather on a longitudinal cutting process. Directional atherectomy is achieved by advancing the deflected catheter tip with the activated cutter through the lesion allowing the spinning blade to shave-off the plaque. It is believed that this novel concept decreases the “Dotter” and balloon angioplasty effect seen with former atheterectomy catheters. Although this technology has been used successfully in human coronary arteries33,34, its main use has been developed to treat peripheral vascular disease35,36.
Intravascular ultrasound
Intravascular ultrasound (IVUS) may be used to identify lesions amenable for DCA, and to examine the extent of plaque removal. In the past, several IVUS studies have suggested that pre-interventional plaque burden is an important determinant of immediate long-term outcome in patients undergoing PCI. This applies to both the pre- and post-stent era. The amount of neointimal hyperplasia in restenotic stent lesions has been shown to correlate with the in initial pre-stent plaque burden. Therefore, pre-intervention IVUS may be a useful to identify patients with large plaque burden, thus providing important triage information for the need of pre-debulking stenting37.
IVUS use is also encouraged to determine the extent of vessel wall calcification and the potential alternate need for rotational atherectomy. The presence of calcium in more than two quadrants by IVUS has become a clear contraindication for DCA. Furthermore, IVUS may be used to guide “optimal” DCA therapy; defined as a residual ultrasound derived plaque burden of less than 50%. It is worthy of mention that the IVUS technology was not available during the initial use of DCA. Thus, it is possible that the majority of DCA landmark clinical trials did not benefit from the valuable sonographic guidance. We must recall that in the subgroup of the AMIGO trial undergoing IVUS guided DCA therapy, significantly better results were observed when compared to the overall study population38. Finally, IVUS has been useful in determining the mechanism of restenosis after DCA39,40.
Contemporary use of DCA therapy
Despite the advent of DES, DCA continues to play a unique role in interventional cardiology. Unfortunately, we have witnessed its use fall dramatically out of favour due to premature and controversial study results. However, we strongly feel that allowing DCA to join the list of extinct interventional tools would be very unfortunate. There are many instances where DCA has been shown to perform superiorly. Below are a few complex cases that serve to illustrate the importance of DCA and its contemporary role as lesion-specific niche device.
Bifurcation lesions are commonly challenging for the interventional cardiologists. Furthermore, despite use of contemporary techniques, they are frequently associated with higher rates of side-branch occlusion and/or restenosis26-28. DCA has be proven useful in these circumstances, and should be highly considered in bifurcating lesions with large reference vessel diameter41, or in those instances where the anticipated plaque shift may result in side-branch occlusion. We here in describe a complex case of a trifurcating left anterior descending coronary artery lesion to illustrate the important applicability of DCA.
Case: A 63 year-old man presented with unstable angina. Coronary angiography revealed tight narrowing in the proximal segment of the left anterior descending coronary (LAD) (Figure 1A); a bulky, non-calcified, highly eccentric lesion adjacent to major septal and diagonal side-branches. Debulking DCA was performed using a 3.0-3.4 mm Flexicut DCA balloon system (Figure 1B).
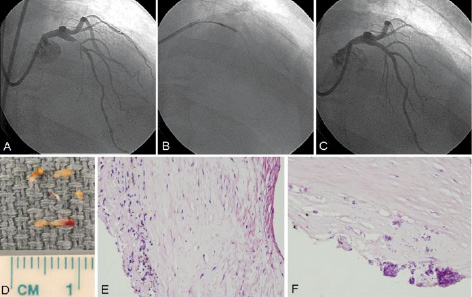
Figure 1. Panel A. Right anterior oblique (RAO) projection showing a bulky and eccentric trifurcating critical lesion involving the proximal left anterior descending (LAD) coronary artery. Panel B. DCA catheter positioned across lesion segment. Panel C. Coronary angiography immediately following successful DCA results. Panel D. Gross ex vivo specimens of atheromatous plaque obtained following DCA. Panel E and F. Corresponding microscopic examination showing fibrosis, inflammation, hemosiderin deposition, and focal dystrophic calcifications are visualised.
A total of 12 consecutive atherectomies were performed using max balloon inflation of 4 atmospheres. Angioplasty was subsequently performed using a 3.5 by 20 mm non-compliant balloon. Successful procedural results are shown in Figure 1C. Histopathological examination of the atherectomised segment is illustrated in Figure 1D-1F. Normal CK-MB levels were recorded during 24-hour follow-up. At 6-months the patient remained asymptomatic and had a normal exercise stress test.
Main branch ostial disease represents another class of lesion in which DCA should be strongly considered29,42, particularly when the presence of a large atheromatous plaque burden may endanger by “snow-plough” effect the adjacent major side-branch. Case number 2 has been described to illustrate this specific scenario.
Case: A 38 year-old male smoker with family history of premature coronary artery disease, and hyperlipidemia presented with non-Q wave myocardial infarction. Diagnostic coronary angiogram demonstrated a bulky non-calcified 80% eccentric lesion in the distal left main coronary artery extending into the ostium of the LAD (Figure 2A).
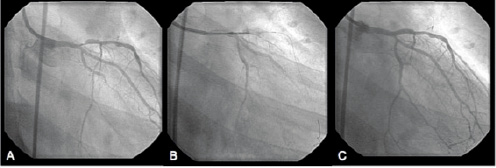
Figure 2. A. Coronary angiography in the right anterior oblique (RAO) projection showed a significant, eccentric stenosis in the ostial left anterior descending artery. B. Flexi-cut catheter is positioned across the lesion. C. Post-procedural angiographic result showed excellent result.
A 95% non-culprit lesion involving the take-off of a small obtuse marginal branch was also appreciated. DCA was performed with the a 2.5-2.9 mm Flexi-cutter that was advanced over the wire from the left main (LM) into the LAD. A total of ten consecutive cuts were performed during minimal balloon inflation (4 atmospheres) (Figure 2B). Following the use of DCA, a paclitaxel stent (3.0x20 mm) was successfully delivered over the LM and proximal LAD stenosis. Final angiographic results following kissing balloon angioplasty are shown in Figure 2C. Routine angiographic follow-up at six months confirmed luminal patency and the absence of in-stent restenosis. The patient remains asymptomatic nine months from his index procedure.
In-stent restenosis (ISR) remains a common problem without a convenient and efficacious treatment. Although multiple strategies have been proposed, only a few have consistently succeeded. We encourage the use of DCA in selected cases with marked in-stent neointimal proliferation43. Atherectomy facilitates in-stent neointimal tissue resection, and further revascularisation. Removal of the in-stent plaque burden potentially decreases the rate of recurrent restenosis and allows for optimal complimentary angioplasty and/ or new stent implantation, particularly (as in the case below) when the use of a DES in a particular patient is prohibitive9,32.
Although the use of DCA for ISR has not been subjected to randomised trials, our initial experience suggests that it is safe and efficacious in bare-metal stents. DCA use in IRS results in large post-procedural lumen diameter, and lower rates of target lesion revascularisation44,45. DCA results in large post-procedural minimal lumen diameter, and low rates of target lesion revascularisation and major follow-up clinical events. Furthermore, DCA appears to be superior to rotablator for in-stent restenosis46. Finally, DCA allows for comparison and characterisation of in vivo tissue histopathology between in-stent and PTCA restenosis47.
Case: A 50 year-old male with poorly controlled hypertension, and coronary artery disease underwent bare metal stenting of his RCA following an inferior myocardial infarction. Ten months later the patient presented with substernal chest pain and exertion dyspnea. Coronary angiography demonstrated the presence of diffuse in-stent restenosis involving the distal RCA stent (Figure 3A). The lesion was treated with a 3.0-3.4 mm DCA Flexicut device. A total of nine cuts were performed at a maximal balloon pressure of 4 atms (Figure 3B). Balloon angioplasty was subsequently performed using a 3.5 by 20 mm non-compliant balloon. Final angiographic results are shown in Figure 3C immediately after the implantation of a 3.5 by 23 mm bare metal stent.
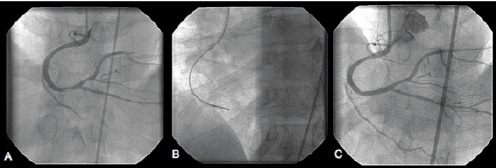
Figure 3. A. Coronary angiography in the anterior posterior cranial projection showing high-grade in-stent restenosis (ISR) in the distal RCA. B. DCA device is positioned across the ISR segment. C. Final coronary angiography showing successful DCA results.
Final thoughts
It is interesting that each device that has been developed for the percutaneous treatment of coronary artery disease has had a similar evolution. Their introduction in clinical practice typically generates great enthusiasm as they are expected to overcome the limitations of prior coronary devices. The first device accepted by the FDA as an alternative to balloon angioplasty was DCA. John Simpson believed this device would solve the major limitations of balloon angioplasty. However, dissolution occurred early on after the initial results of randomised control trials showing similar immediate results to balloon angioplasty and worsening late outcome. When dissolution occurs, device indications are placed in perspective, and their advantage, limitations and safety are better understood. As a result, they are often set aside as a “niche” device. High speed rotational atherectomy, intracoronary laser, and thrombectomy devices among others represent a clear example.
Therefore, we strongly feel that DCA should remain as a niche device in the armamentarium of interventional cardiology. However, handling the training of new generation interventional cardiologists and credentialing their competency will constitute a challenge particularly in an environment where DCA is rarely used. Furthermore, during our present cost-conscious medical environment the cost-benefit of adjunctive DCA needs to be individualised. Nevertheless, DCA is distinctively helpful when treating ostial coronary lesions, intricate bifurcations, and lesions with large plaque burden. Thus we believe, particularly after the results of the START trial that its use should be highly entertained in selected complex cases and in patients not considered candidates for DES implantation due to possible problems with prolonged dual antiplatelet therapy. We thus hope that the manufacturers of DCA continue its production and preserve this valuable tool as a “niche” device for the well-being of our patients.
Acknowledgements
Disillusion to DCA therapy occurred soon after the discouraging results of the CAVEAT 1 trial. However, thanks to the audacious work of a group of interventional cardiologist that believed in the device, and its conceptual value it exceeded its initial disappointing results. Dr. Donald Baim has been a world renowned cardiologist and a major contributor of DCA expansion. The authors would like to fully acknowledge Dr. Donald Baim for his careful review, expert opinion, and priceless editorial contribution to this review.

