Abstract
Aims: Angiographic analysis of left main coronary artery (LMCA) stenosis can be hindered by the lack of any reference segment when the LMCA is short or there is diffuse atheroma. Fractal geometric law (FGL) enables the theoretic diameter of one bifurcation vessel to be calculated from those of the other two (Dmother=0.678*(Ddaughter1+Ddaughter2). Applied to the LMCA, the FGL can help the quantification of stenoses.
Methods and results: Fifty-two patients with angiographically mild focal LMCA disease (n=14) or normal to nearly normal LMCA (n=38) who had undergone intravascular ultrasound (IVUS) were included. IVUS analysis confirmed all 14 focal stenoses (group C); of the 38 angiographically normal patients, however, 10 were found to present diffuse LMCA disease (group B), the remaining 28 showing a truly healthy LMCA (group A). LMCA stenosis in groups A,B and C was respectively 3%,4% and 42% on usual quantitative coronary angiography(QCA) and 5%, 31% and 43% on QCAfractal applying the FGL. In cases of diffuse atheroma, the FGL corrected the underestimation of LMCA diameter, which averaged 1.2 mm.
Conclusions: Angiographic underestimation of LMCA stenosis can be corrected by applying the FGL to obtain a theoretic LMCA diameter, thereby unmasking any diffuse atherosclerotic LMCA disease, or to quantify focal stenosis more precisely where the adjacent segments are also pathological.
Introduction
The spontaneous prognosis of severe left main coronary artery (LMCA) stenosis (37% survival at three years1) is of sufficient concern to make a correct diagnosis crucial. Angiography, however, presents many limitations in assessing LMCA disease2. The main problem is in the case of diffuse atherosclerotic involvement and the absence of a normal reference segment, especially when the LMCA is short3. Stenosis diameter is then difficult to calculate and is often underestimated4. Other techniques such as intravascular ultrasound (IVUS) often need to be called upon to confirm the degree of stenosis and assess the need for revascularisation5. Although IVUS is the reference examination in assessment and treatment guidance for angiographically indeterminate LMCA disease6, it is still not being systematically used for this indication unless angiography specifically suggests possible LMCA disease, and is not available in all cathlabs.
The present study sought to apply results based on the fractal geometry of coronary bifurcations to LMCA angiography, with a view to enhancing the detection and quantification of diffuse atherosclerotic LMCA disease. We confirmed the fractal nature of the geometry of the epicardial coronary artery tree, as well as providing a simple and accurate fractal ratio between the diameters of the mother and two daughter vessels such that Dmother= 0.678*(Ddaughter1+Ddaughter2). This makes it easy to calculate the precise diameter of any of the three vessels when those of the other two are known7. Applied to the LMCA, this fractal geometric law (FGL) provides the true LMCA reference diameter from those of the left anterior descending (LAD) and left circumflex (LCx) coronary arteries, enabling the true percentage stenosis to be calculated.
An angiographic and IVUS study was performed on patients whose LMCA was considered normal or nearly normal, and on patients presenting intermediate focal stenosis of the LMCA, with a view to validating the interest of the FGL in LMCA analysis.
Methods
Study population
Fifty-two patients who had undergone quantitative coronary angiography (QCA) and complete left coronary tree IVUS exploration, either for angina diagnosis or following acute coronary syndrome, were included. All patients signed an informed written consent form, and the study was approved by the institution’s Ethics Committee.
Procedure
Coronary angiography was performed using a digital flat-panel detector (INNOVA®; GE Healthcare Ltd, Little Chalfont, Buckinghamshire, United Kingdom) with a femoral artery approach, with insertion of a 6 Fr sheath and a guiding catheter of the same size. The incidences were adapted to show up the LMCA and proximal LAD and LCx segments in two orthogonal planes. Before IVUS examination, patients received low-dose IV heparin (30 U/kg). Intracoronary nitroglycerin was administered to avoid coronary spasm. The IVUS imaging protocol was performed as described previously8, using a Galaxy® digital imaging console (Boston Scientific Corp., San Jose, CA, USA). After angiography, a 0.014-inch guidewire was passed through the 6 Fr guiding catheter from the LMCA to the LAD then LCx arteries. The IVUS transducer (mechanical scanning IVUS catheter, Atlantis 40 MHz) was inserted up to a point beyond the bifurcation from the LMCA to the LAD then LCx. Pullback images were recorded, using a motorised system (1 mm per second).
Group selection
On the basis of angiography, two independent operators distinguished two populations:
– normal or nearly normal LMCA: LMCA with parallel or nearly parallel edges on all incidences, without significant atherosclerotic irregularity and <20% diameter stenosis on QCA;
– mild to moderate LMCA stenosis: reduced focal caliber on at least one incidence; >20% diameter stenosis on QCA.
IVUS classified the 52 LMCAs into three groups: Group A– Normal LMCA, with plaque burden <40% along the entire length; Group B– Diffuse LMCA disease, with plaque burden >40% along the entire length; Group C– Focal LMCA disease, focal stenosis with plaque burden >40% in an otherwise normal or nearly normal LMCA.
Quantitative angiographic and IVUS analyses were compared in each group.
Quantitative coronary angiography analysis
Quantitative coronary angiography was performed off-line, using computer-assisted edge-detection methods (INNOVA®; GE Healthcare Ltd, Little Chalfont, Buckinghamshire, United Kingdom). The contrast-filled 6 Fr catheter was used for calibration. The minimal lumen and reference diameters of the LMCA were measured in diastolic frames obtained by angiography before IVUS examination. Percentage diameter stenosis was calculated. The diameters of the proximal LAD and LCx segments before the first collateral branch were measured with the same method.
Quantitative IVUS analysis
The quantitative analyses were performed offline by an investigator blinded to the QCA findings, using the INDEC® workstation (TapeMeasure, INDEC Systems, Mountain View, CA, USA), according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies9. For each site, four parameters were measured: lumen cross-sectional area (CSA), obtained by computed planimetry of the intimal leading edge; external elastic membrane (EEM) cross-sectional area; plaque-plus-media (P+M) cross-sectional area, calculated as the difference between EEM and CSA; and plaque burden (PB%), calculated as the ratio of P+M to EEM (PB%=P+M/EEMx100). The sites analysed were the proximal LAD, the proximal LCx, and the LMCA reference and lesion sites (respectively, the sites where the lumen area was greatest and smallest). Vessel diameters were calculated from the IVUS areas, as d=2√CSA/∏.
The fractal geometric law (FGL)
Coronary artery bifurcations present a harmonious asymmetric geometry that is fractal in nature. Finet et al.7 confirmed the fractal nature of pericardial coronary arterial tree geometry and provided a simple fractal ratio relating mother-vessel diameter (Dm) and the daughter-vessel diameters (Dd) by the formula: Dm=0.678* (Dd1+Dd2). These calculations proved exact – more so than those derived from Murray’s law or the law of conservation of flow, which moreover are much more complex in their formulations. The present study applied FGL to derive a theoretic LMCA diameter (LMDfractal) from the measured reference diameters of the left anterior descending (LADDref) and circumflex (LCxDref) coronary arteries. Each reference diameter was located in an artery segment adjacent to the bifurcation (first 10 mm) in optimised coronary angiographic views.
The formula, applicable to angiographic and to IVUS measurements, was:
LMDfractal= 0.678*(LADDref+LCxDref)
Statistical analysis
The three groups of patients distinguished on IVUS were compared in regard to several characteristics. For categorical variables, data were displayed through contingency tables and a Fisher exact test was carried out. For continuous variables, results were displayed as mean±standard error (SE) and a one-way analysis of variance (ANOVA) was used to test the overall group effect. When appropriate (i.e., in case of prior significant ANOVA), post-hoc multiple comparison was computed, through a Tukey Honestly Significant Difference (THSD) test, to detail results controlling for type I error. All statistical analyses were carried out on SAS software (SAS v9.1, SAS Institute Incorporation, Cary, NC, USA) with 0.05 type-I error.
Results
Details of the 52 subjects are given in Table 1.
On angiography, 38 LMCAs were assessed as normal or nearly normal, and 14 as bearing mild to moderate stenosis. IVUS confirmed these 14 focal stenoses (group C); 10 of the angiographically normal or nearly normal LMCAs, however, were found to present diffuse atheroma (group B), the other 28 being truly healthy (group A). Each of these three IVUS groups is illustrated by 3 quantitative examples (Figure 1).
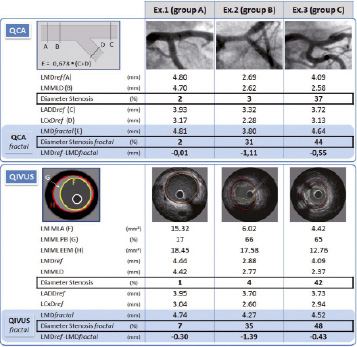
Figure 1. Examples of normal LMCA (Ex. 1), diffuse LMCA disease (Ex. 2) and focal LMCA disease (Ex. 3). QCA analysis: reference left main diameter (LMDref); left main minimal lumen diameter (LMMLD); reference left anterior descending diameter(LADDref); reference circumflex diameter (LCxDref). QIVUS analysis: Measurements of left main where the lumen is minimal (left main minimal lumen area (LMMLA), left main minimal lumen plaque burden (LMML PB), left main minimal lumen elastic external membrane area (LMML EEM). Diameters calculated from cross-sectional area by d=2√CSA/∏ for reference left main, left main minimal lumen, LAD and LCx (LMDref, LMMLD, LADDref, LCxD ref). Calculation of theoretic LMCA diameter on QCA and QIVUS by fractal geometric law: (LMDfractal=0.678* (LADDref+ LCxDref). Stenosis diameter (%) as obtained by relating LMMLD to LMDref (stenosis diameter) or LMDfractal (fractal stenosis diameter). Difference between measured and theoretic LMCA diameters.
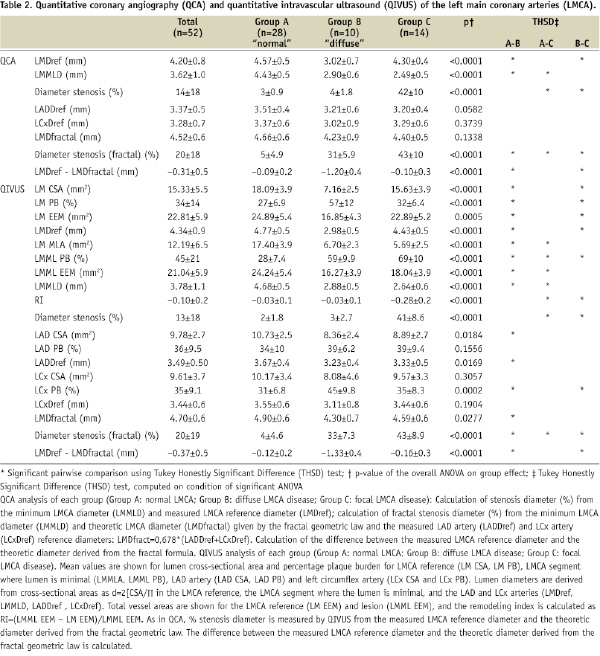
Percentage stenosis diameter in groups A, B and C was respectively 3%, 4% and 42% on usual QCA and 2%, 3% and 41% on usual QIVUS (Table 2); applying the FGL, stenosis was respectively 5%, 31% and 43% on QCA and 4%, 33% and 43% on QIVUS.
Diffuse atherosclerotic LMCA disease was unmasked by the FGL in case of stenosis diameters exceeding a 20% cutoff value, mean percentage stenosis rising from 4% to 31% in this group (Figure 2).
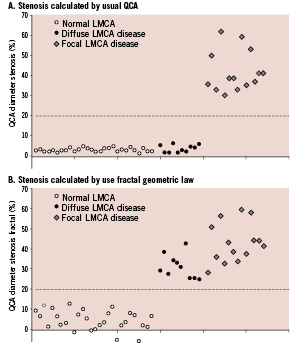
Figure 2. Diffuse atherosclerotic left main coronary artery disease unmasked by application of fractal geometric law to quantitative coronary angiography. Percentage stenosis diameter calculated from the minimum and reference LMCA diameters on QCA (A) or from the minimum and theoretic LMCA diameters (B). The theoretic diameter is derived from the fractal geometric law and the LAD and LCx reference diameters.
In LMCAs that were normal or presented focal stenosis, there was no significant difference in reference diameter as measured directly and as calculated by the fractal formula from the measured LAD and LCx diameters (Figure 3), validating the FGL on both QCA and QIVUS for normal and focally lesioned LMCAs. The FGL unmasked diffuse LMCA disease (group B), where the degree of stenosis was underestimated by usual QCA. In case of diffuse atherosclerotic LMCA disease, the mean underestimation on angiographic measurement as compared to the theoretic diameter calculated from the FGL was 1.20 mm on QCA and 1.33 mm on QIVUS.
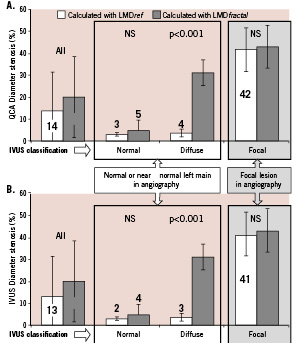
Figure 3. Normal LMCA and reference segments in focal LMCA disease conform to fractal geometry. Interest of the fractal geometric law in unmasking diffuse LMCA disease. Percentage LMCA stenosis calculated on QCA (A) from minimum diameter and either measured reference diameter or theoretic diameter as given by the fractal geometric law. Percentage LMCA stenosis calculated on QIVUS (B) from minimum diameter and either measured reference diameter or theoretic diameter as given by the fractal geometric law. Values are shown for the whole sample (n=52), and the normal LMCA (n=28), diffuse LMCA disease (n=10) and focal LMCA disease (n=14) groups.
Nine of the 38 LMCAs (24%) appearing normal or nearly normal on angiography showed stenosis (MLA<9 mm2) on IVUS, with MLA<6 mm2 in three cases (8%). Applying the fractal law, only 28 angiographies were still considered normal (Figure 4). The negative predictive value of the FGL was 100%, these 28 LMCAs all presenting MLA>9 mm2 on IVUS, an index known to be of good prognosis. 15 of the 24 LMCAs assessed as pathological by FGL were revascularised by bypass or percutaneous intervention (63%).
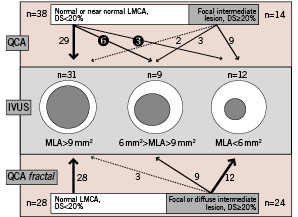
Figure 4. Prognostic value of usual QCA and QCA calculated with fractal geometric law (QCAfractal) in unmasking LMCA lesions precisely quantified on IVUS as mild (MLA> 9 mm2, n=31), intermediate (9 mm2 < MLA < 6 mm2, n=9) or severe (MLA <6 mm2, n=12). In usual QCA, 9/38 of normal or nearly normal LMCAs (24%) had MLA <9 mm2, and 3/38 had MLA <6 mm2 (8%).The negative predictive value of fractal QCA was excellent: 100% of the 28 normal LMCAs showed MLA>9 mm2 on IVUS.
Discussion
The fractal geometric law was validated for the assessment of coronary bifurcations7. With this simple linear law, the theoretic diameter of one arterial segment of a coronary bifurcation can be derived from the diameters of the other two. The present study extends the application of this law to the left main coronary artery (LMCA). In LMCAs confirmed as healthy on IVUS, there was no significant difference in the diameter as measured and as theoretically calculated using the FGL, and the same was true of healthy LMCA segments on either side of focal stenoses. To the best of our knowledge, only one study, on just 20 LMCA angiograms10, has quantified the ratio between the sum of the daughter vessel diameters and the mother vessel diameter; the ratio came out at 0.65±0.04 – very close to that found by Finet et al7. Applying this law to the LMCA gets round one of the major problems of angiographic assessment of stenosis, which is the lack of reference segment. Our IVUS study confirmed that the minimum measured diameter is to be assessed against a new reference value given by: LMDfractal= 0.678*(LADref+LCxDref). The FGL is a simple means of unmasking diffuse atherosclerotic disease in the LMCA or of avoiding underestimation of a focal lesion as seen on the angiogram. In our study, the prognostic value of angiography was excellent after application of the FGL. For an angiographic stenosis diameter of <20% with respect to the theoretic LMCA diameter given by the FGL, the minimum lumen area on IVUS was consistently greater than 9 mm2. The negative predictive value of fractal QCA was 100% in the present series, none of the 28 LMCA assessed as normal needing revascularisation in the light of the IVUS findings.
Coronary stenosis is routinely assessed by angiography and visual or computerised quantification of narrowing with respect to an adjacent reference segment deemed to be normal, with stenosis being counted as severe in case of a stenosis diameter of >50%. The limitations of angiography in the assessment of LMCA disease are, however, well known and confirmed by anatomic imaging from histopathological specimens and IVUS studies4. There is significant inter- and intra-observer variability in stenosis quantification on coronary angiography11. Moreover, angiography is just a 2-dimensional X-ray projection of the contrast-filled arterial lumen. Stenoses might be incorrectly assessed in case of an excentric lesion, tortuosity or angulation of the coronary arteries, vessel overlap or bifurcation. These known limitations are frequently encountered in LMCA analysis, especially in case of a short vessel with possible diffuse atheroma and when no disease-free reference segment is available. Underestimating an LMCA stenosis, however, can be a serious matter. The CASS registry reported 13.3 years’ median survival in their surgical group, compared to 6.6 years in the medical group (p<0.0001) for LMCA with stenosis diameter >50% on angiography12.
IVUS provides high-resolution cross-sectional images of the coronary lumen and arterial wall. Several studies have shown the interest of IVUS in case of ambiguity on angiography and in particular in the assessment of intermediate stenoses, bifurcation lesions and LMCA disease2,8. Angiography’s limitations in LMCA assessment explain the poorer correlations with IVUS found in this area13,14. IVUS is now the recognised reference examination for LMCA disease assessment and treatment guidance. The prognostic value of minimal lumen area (MLA) on IVUS guides revascularisation or continuation of medical management. According to Nissen8, an LMCA with stenosis area >50% or absolute area <9 mm2 are stenosis severity criteria for revascularisation8. Abizaid et al3, in a cohort of 122 cases of moderate LMCA disease, reported a one-year event rate of 14%. The most important quantitative predictor of cardiac events was the minimal lumen diameter (MLD) as measured on IVUS. The event rate was just 3% for MLD >3 mm (corresponding to MLA>7.1 mm2 in a circular lumen), reaching 60% for MLD<2 mm. Fassa et al6 studied the efficacy of IVUS to guide strategy for patients with angiographically indeterminate LMCA disease. In a cohort of 121 patients, the mean MLA was 16.25±4.30 mm2. The authors proposed an MLA cut-off of 7.5 mm2 as a decision criterion to proceed with or delay revascularisation; between 7.5 mm2 and 9.6 mm2 they speak of a “grey zone” where other factors such as age, smoking status and extent of coronary disease are also to be taken into account. For Jasti et al15,16, MLA>5.9 mm2 is strong predictor of survival and event-free survival rates. In the most recent studies5,17, the MLA cut-off defining severe LMCA stenosis requiring revascularisation is 6 mm2; between 6 and 7.5 mm or 9 mm2, stenosis is intermediate and treatment should be guided by additional parameters.
Although indispensable for the assessment of angiographically ambiguous LMCAs, IVUS is still not widely used. IVUS consoles and experimented operators are not available in all centres; and even where available, IVUS is not always deployed where the angiogram fails to suggest stenosis by diffuse atheroma.
Simple application of the FGL can alert the operator in case of diffuse atheroma and of additional focal stenosis. In the present series, three “pure” groups were investigated: normal, or diffuse or focal LMCA disease. In practice, intermediate focal stenoses are often found within diffuse atheroma. The FGL, defining a new reference diameter, can correct the angiographic underestimation of such stenoses reported in comparative studies against IVUS14. In our series, which did not include any severe stenoses, LMCA diameter was underestimated by a mean 1.20 mm in case of diffuse LMCA disease. Finally, moderate obstructive LMCA disease not detected by angiography but evident on IVUS is associated with future cardiac events and poor prognosis18. The FGL could help to detect early atherosclerotic LMCA disease and to guide patient management and follow-up.
Several lessons are to be drawn from the present validation of FGL in healthy and pathological LMCA analysis. Angiography underestimates the degree of LMCA stenosis in case of diffuse atheroma. The FGL unmasks such diffuse stenosis and also improves the quantification of associated focal lesions. It confirms IVUS findings on coronary diameters either side of a bifurcation. Fractal geometry entails a difference of at least 1 mm between proximal LAD and LMCA diameters. Now that angioplasty offers an alternative to bypass grafting in the management of LMCA stenosis19, this is an important point to be taken into account, requiring a stent diameter adapted to the daughter-vessel diameter for provisional T-stenting of a bifurcation, with proximal postdilatation by final kissing balloon so as to avoid malapposition in the mother vessel.
Study limitations
Applying FGL requires two healthy segments so as to derive the theoretic diameter of the third. Atheroma extending into the daughter vessels may induce an underestimation of the mother-vessel diameter and hence of the degree of stenosis, which the FGL is intended to correct. In the present series, the absence of LAD and LCx stenosis on angiography was confirmed by IVUS. This limitation, however, remains for any QCA stenosis assessment inasmuch as the so-called reference segment providing the minimal diameter is not always in fact healthy: Mintz et al21 showed that the reference segment proved healthy on IVUS in only 6.8% of cases. Even so, QCA is the tool routinely used, and is the reference in most clinical trials.
The second limitation concerns the impossibility of applying the FGL in the case of a trifurcation.
Conclusion
Applying the linear law describing the fractal geometry of arterial bifurcations to the angiographic quantification of a left main coronary bifurcation enables the real reference diameter to be approximated. This can unmask diffuse atherosclerotic left main coronary artery disease on quantitative coronary angiography. Moreover, focal LMCA stenoses, which tend to be easily detectable, can be more precisely quantified, avoiding underestimation due to an adjacent atherosclerotic segment.

