Abstract
Background: Coronary artery perforation is a feared complication of chronic total occlusion (CTO) percutaneous coronary intervention (PCI) and often leads to serious adverse clinical events.
Aims: We sought to develop a risk score to predict clinical coronary artery perforation in patients undergoing CTO PCI.
Methods: We analysed clinical and angiographic parameters from 9,618 CTO PCIs in the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS-CTO). Logistic regression prediction modelling was used to identify variables independently associated with clinical perforation, and the model was internally validated with bootstrapping. Clinical coronary artery perforation was defined as any perforation requiring treatment.
Results: The incidence of clinical coronary perforation was 3.8% (n=367). Five factors were independently associated with perforation and were included in the score: patient age ≥65 years +1 point (odds ratio [OR] 1.79, 95% confidence interval [CI]: 1.37-2.33), moderate/severe calcification +1 point (OR 1.85, 95% CI: 1.41-2.42), blunt/no stump +1 point (OR 1.45, 95% CI: 1.10-1.92), use of antegrade dissection and re-entry +1 point (OR 2.43, 95% CI: 1.61-3.69), and use of the retrograde approach +2 points (OR 4.02, 95% CI: 2.95-5.46). The resulting score showed acceptable performance on receiver operating characteristic (ROC) curve (area under the curve [AUC]: 0.741, 95% CI: 0.712-0.773). The Hosmer-Lemeshow test indicated a good fit (p=0.991), and internal validation with bootstrapping demonstrated good agreement with the model with observed AUC: 0.736 (95% bias-corrected CI: 0.706-0.767).
Conclusions: The PROGRESS-CTO perforation score may be a useful tool for predicting clinical coronary perforation during CTO PCI.
Introduction
Coronary artery perforation is an infrequent but potentially life-threatening complication of percutaneous coronary intervention (PCI), as it can lead to pericardial effusion and tamponade123. The incidence of perforation is higher in chronic total occlusion (CTO) PCI, likely due to higher lesion complexity and use of advanced crossing strategies4. Several factors have been associated with an increased risk of perforation during CTO PCI, such as age, prior coronary artery bypass graft surgery (CABG), proximal cap ambiguity and use of the retrograde approach or antegrade dissection and re-entry (ADR)56. We developed a scoring system to predict the occurrence of clinical coronary artery perforation during CTO PCI.
Methods
Patient population
We examined the clinical, angiographic, and procedural characteristics of 9,618 CTO PCIs performed between 2012 and 2021 at 38 centres: Unites States (33), Canada (1), Europe (1), Turkey (1), Egypt (1) and Russia (1) (Supplementary Table 1). Data were recorded in a dedicated online database (PROGRESS-CTO: Prospective Global Registry for the Study of Chronic Total Occlusion Intervention; ClinicalTrials.gov: NCT02061436). Study data were collected and managed using Research Electronic Data Capture (REDCap; Vanderbilt University) electronic data capture tools hosted at the Minneapolis Heart Institute Foundation78. Patients who had a second CTO PCI during the sudy period were excluded from the analysis, as were procedures without data on technical success, procedural success, or periprocedural complications. The study was approved by the institutional review board of each centre.
Definitions
Coronary CTOs were defined as coronary lesions with a Thrombolysis in Myocardial Infarction (TIMI) grade 0 flow of at least 3 months in duration. Calcification was assessed by angiography as mild (spots), moderate (involving ≤50% of the reference lesion diameter), or severe (involving >50% of the reference lesion diameter). Blunt or no stump was defined as a lack of tapering or lack of a funnel shape at the proximal cap. Interventional collaterals were defined as collaterals considered amenable to crossing by a guidewire and a microcatheter by the operator. Clinical coronary artery perforation was defined as any perforation requiring treatment. For risk calculation purposes, antegrade wiring (AW) was defined as no utilisation of either ADR or retrograde crossing attempts. If AW and ADR were both used, the case was classified as ADR. If a retrograde strategy was used, the crossing strategy was defined as retrograde.
Technical success was defined as successful CTO revascularisation with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. Procedural success was defined as the achievement of technical success without any in-hospital major adverse cardiac events (MACE). In-hospital MACE included any of the following adverse events prior to hospital discharge: death, myocardial infarction (MI), recurrent symptoms requiring urgent repeat target vessel revascularisation with PCI or CABG surgery, tamponade requiring either pericardiocentesis or surgery, and stroke. The Japanese Multicentre CTO Registry (J-CTO) score was calculated as described by Morino et al9 and the PROGRESS-CTO score as described by Christopoulos et al10.
Statistical analysis
Categorical variables were presented as absolute numbers and percentages and were compared using Pearson’s chi-square test or Fisher’s exact test, as appropriate. Continuous variables were presented as mean±standard deviation or median (interquartile range [IQR]) unless otherwise specified and were compared using the Student’s t-test or the Wilcoxon rank-sum test, as appropriate. Univariable logistic regression was performed to identify associations between variables and outcome (clinical coronary artery perforation). Variables that had a p-value of <0.10 in univariable analysis and were considered clinically/angiographically plausible predictors of coronary perforation were included in multivariable logistic regression with backward elimination, starting with variables that had the highest p-value. All dropped variables that were considered clinically/angiographically plausible were then individually added to investigate any other potential confounders by checking the change in the beta coefficients in the multivariable regression model. The Hosmer-Lemeshow test was used to test goodness of fit withp>0.05 considered a good fit. The discriminative capacity of the model was illustrated with receiver operating characteristic (ROC) curve analysis, with areas under the ROC curve (AUC) 0.70-0.80 and 0.80-0.90 considered to have an acceptable and excellent discrimination, respectively. Internal validation was performed with bootstrapping 1,000 samples from the dataset. A calculator incorporating the variables in the final prediction model was created (Supplementary Table 2) by calculating the probability of event (P) with the logistic regression equation: ln(P/[1-P])= β0 + β1*x1 + ... + βz*xz. To facilitate clinical use and for risk score assignment, age was transformed into a categorical variable and a binary risk score was provided. The risk score was created from the full prediction model by assigning weighted points to the beta coefficients in the final model. Temporal trends were tested for significance with linear contrast analysis (for continuous variables) and with the Cochran-Armitage test (for categorical variables). All statistical analyses were performed using JMP, version 14.0 (SAS Institute) and Stata v.17.0 (StataCorp). A p-value of <0.05 was considered statistically significant.
Results
Baseline characteristics and procedural outcomes
The baseline clinical and angiographic characteristics and procedural outcomes in the perforation and no perforation groups are summarised in Table 1. Patients who experienced coronary perforation were older; had a higher prevalence of hypertension, dyslipidaemia, and peripheral arterial disease; and were more likely to have had prior PCI and prior CABG. Lesions that had perforation were longer (34±21 mm vs 30±20 mm; p<0.001) and more likely to have unfavourable characteristics, such as proximal cap ambiguity (53% vs 34%; p<0.001), blunt proximal cap (58% vs 46%; p<0.001), moderate or severe proximal vessel tortuosity (41% vs 29%; p<0.001), and moderate or severe calcification (70% vs 46%; p<0.001). The J-CTO score (3.0±1.2 vs 2.3±1.3; p<0.001) and the PROGRESS-CTO score (1.5±1.0 vs 1.2±1.0; p<0.001) were higher in the perforation group.
AW was the most common successful crossing strategy in the no perforation group (56% vs 21%; p<0.001) and the retrograde approach (38% vs 19%; p<0.001) was the most frequent successful crossing strategy in perforation cases. Technical (70% vs 87%; p<0.001) and procedural (56% vs 86%; p<0.001) success rates were lower among perforation cases. Patients with perforation more often required use of left ventricular assist devices (12% vs 4%; p<0.001) and had longer hospital stays after CTO PCI (2.9±5.3 vs 1.5±2.5 days; p<0.001). The incidence of MACE was higher in perforation cases (21% vs 1.4%; p<0.001).
Table 1. Clinical, angiographic, and procedural characteristics of patients with and without clinical coronary perforation.
| Variables | Perforation (n=367, 3.8%) | No perforation (n=9,251, 96.2%) | p-value | |
|---|---|---|---|---|
| Age (years), mean±SD (n) | 69±10 (325) | 64±10 (8,520) | <0.001 | |
| Men, n (%) | 259 (79) | 7,071 (81) | 0.177 | |
| BMI (kg/m2), mean±SD (n) | 30±5 (281) | 31±6 (7,905) | 0.016 | |
| Hypertension, n (%) | 305 (94) | 7,650 (90) | 0.005 | |
| Dyslipidaemia, n (%) | 297 (93) | 7,393 (87) | 0.002 | |
| Diabetes mellitus, n (%) | 128 (41) | 3,628 (43) | 0.548 | |
| Prior MI, n (%) | 149 (48) | 3,657 (45) | 0.239 | |
| Prior PCI, n (%) | 249 (72) | 5,389 (62) | <0.001 | |
| Prior CABG, n (%) | 156 (44) | 2,516 (29) | <0.001 | |
| Congestive heart failure, n (%) | 95 (30) | 2,388 (29) | 0.444 | |
| Peripheral arterial disease, n (%) | 75 (23) | 1,168 (14) | <0.001 | |
| Cerebrovascular disease, n (%) | 44 (14) | 839 (10) | 0.022 | |
| LVEF (%), mean±SD (n) | 50±13 (304) | 50±13 (7,509) | 0.796 | |
| Baseline creatinine (mg/dl), mean±SD (n) |
1.1±0.4 (269) | 1.1±0.5 (7,707) | 0.826 | |
| CTO target vessel | RCA, n (%) | 210 (59) | 4,631 (53) | 0.038 |
| LAD, n (%) | 78 (21) | 2,317 (26) | 0.045 | |
| LCx, n (%) | 63 (17) | 1,680 (19) | 0.453 | |
| Other, n (%) | 9 (3) | 179 (2) | 0.237 | |
| Successful crossing strategy | AW, n (%) | 76 (21) | 5,072 (56) | <0.001 |
| ADR, n (%) | 66 (18) | 1,188 (13) | ||
| Retrograde, n (%) | 139 (38) | 1,694 (19) | ||
| None, n (%) | 86 (23) | 1,157 (12) | ||
| Calcification (moderate-severe), n (%) | 241 (70) | 3,871 (46) | <0.001 | |
| Blunt/no stump, n (%) | 214 (58) | 4,289 (46) | <0.001 | |
| IVUS, n (%) | 165 (62) | 3,385 (46) | <0.001 | |
| ADR: antegrade dissection and re-entry; AW: antegrade wiring; BMI: body mass index; CABG: coronary artery bypass graft surgery; CTO: chronic total occlusion; IVUS: intravascular ultrasound; LAD: left anterior descending; LCx: left circumflex; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; RCA: right coronary artery; SD: standard deviation | ||||
Type, mechanisms and treatment of perforations
The type, severity and mechanisms of clinical perforations are shown in Table 2. The incidence of clinical coronary perforation was 3.8% (n=367) and did not significantly change over time (p for trend=0.354) (Supplementary Figure 1). The perforations that occurred during retrograde crossing attempts were epicardial collateral perforations in 34%, septal collateral perforations in 22% and large vessel perforations in 35%. Perforations that occurred during ADR were large vessel perforations in 72% and distal vessel perforations in 28%. Half of AW perforations were large vessel and the other half were distal vessel perforations (Supplementary Table 3).
The mechanism of clinical perforation in AW cases was guidewire exit in 60% and balloon inflation in 25%. In ADR cases the most common mechanism of perforation was guidewire exit (52%), followed by balloon inflation (15%) and the CrossBoss (Boston Scientific) catheter (11%). In retrograde perforations, the most common cause was guidewire exit (46%), followed by balloon inflation (20%), microcatheter advancement (17%) and stenting (11%).
The treatment of the perforations is summarised in Table 3. In 103/367 (28%) of cases only anticoagulation reversal was used; the remaining 72% of perforations required one or more interventions to treat the perforation. The management of perforations stratified by perforation type is illustrated in Figure 1.
Table 2. Clinical coronary artery perforation characteristics.
| Perforation type | |
|---|---|
| Large vessel, n (%) | 146 (40) |
| Distal vessel, n (%) | 96 (26) |
| Septal collateral, n (%) | 26 (7) |
| Epicardial collateral, n (%) | 40 (11) |
| Unknown, n (%) | 59 (16) |
| Crossing strategy caused perforation | |
| AW, n (%) | 133 (36) |
| ADR, n (%) | 46 (13) |
| Retrograde, n (%) | 118 (32) |
| Unknown, n (%) | 70 (19) |
| Perforation severity | |
| Ellis Class 1, n (%) | 61 (17) |
| Ellis Class 2, n (%) | 121 (33) |
| Ellis Class 3, n (%) | 109 (30) |
| Ellis Class–cavity spilling, n (%) | 26 (7) |
| Unknown, n (%) | 50 (13) |
| Mechanism of perforation | |
| Guidewire exit, n (%) | 173 (47) |
| Balloon inflation, n (%) | 71 (19) |
| Microcatheter advancement, n (%) | 40 (11) |
| Stenting, n (%) | 27 (7) |
| CrossBoss catheter, n (%) | 5 (1.5) |
| Rotational atherectomy, n (%) | 2 (0.7) |
| Orbital atherectomy, n (%) | 1 (0.3) |
| Laser atherectomy, n (%) | 3 (1) |
| Other, n (%) | 5 (1.5) |
| Unknown, n (%) | 40 (11) |
| ADR: antegrade dissection and re-entry; AW: antegrade wiring | |
Table 3. Treatment of cases that resulted in clinical perforation.
| Perforation treatment | n=367 |
|---|---|
| Anticoagulation reversal, n (%) | 103 (28) |
| Prolonged balloon inflation, n (%) | 115 (31) |
| Covered stent, n (%) | 106 (29) |
| Coil embolisation, n (%) | 30 (8) |
| Fat embolisation, n (%) | 18 (5) |
| Pericardiocentesis, n (%) | 69 (19) |
| Emergency surgery, n (%) | 4 (1) |
| Other, n (%) | 24 (7) |
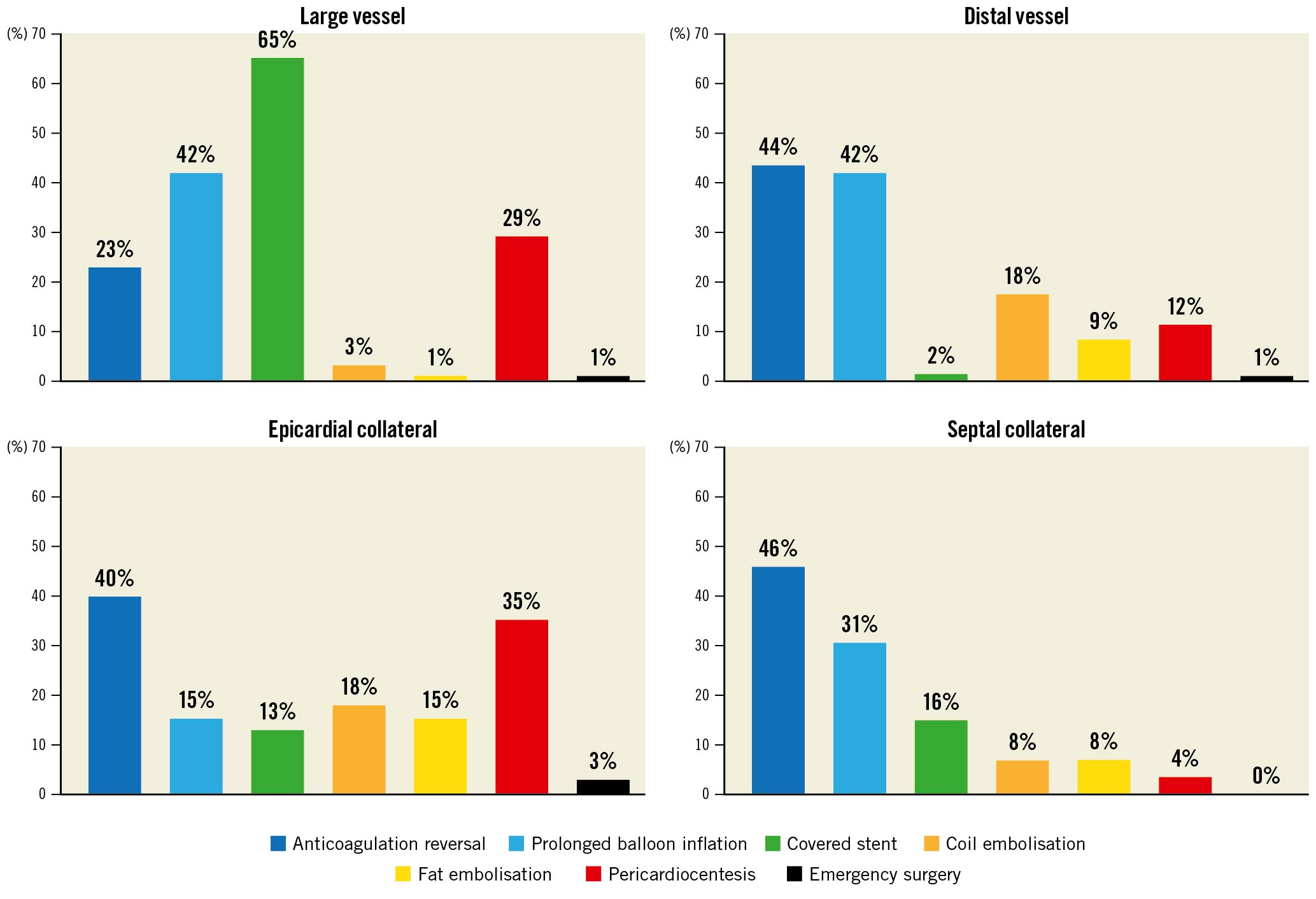
Figure 1. Coronary perforation treatment stratified by perforation type.
Perforation risk prediction model
On univariable logistic regression, age, prior CABG, right coronary artery (RCA) CTO, moderate-severe calcification, moderate-severe proximal vessel tortuosity, proximal cap ambiguity, blunt/stumpless proximal cap, ADR use, and use of the retrograde approach were associated with clinical perforation with a p-value <0.10.
A multivariable logistic regression model to predict clinical coronary artery perforation during CTO PCI was built with these variables, and the final model was created as described in the methods. The final model included 1) age (continuous), 2) use of ADR (yes/no), 3) use of the retrograde approach (yes/no), 4) moderate/severe calcification (yes/no), 5) blunt/stumpless proximal cap (yes/no) (Figure 2). The PROGRESS-CTO perforation risk calculator for clinical use was created with these variables (Supplementary Table 2, Supplementary Table 4). The PROGRESS-CTO perforation risk score showed acceptable performance on ROC analysis (AUC: 0.741, 95% confidence interval [CI]: 0.712-0.773) (Figure 3). The calibration plot showed good agreement between the observed and expected rates of coronary perforation (Figure 4). The Hosmer-Lemeshow test indicated good fit (p=0.991), and internal validation with bootstrapping of 1,000 samples demonstrated good agreement with the model (observed AUC: 0.736, 95% bias-corrected CI: 0.706-0.767).
Age was dichotomised (<65 years and ≥65 years) and risk points were assigned to each variable based on the magnitude of the odds ratio (OR): +1 point for age ≥65 years (OR 1.79, 95% CI: 1.37-2.33); +1 point for use of ADR (OR 2.43, 95% CI: 1.61-3.69); +2 points for use of the retrograde approach (OR 4.02, 95% CI: 2.95-5.46); +1 point for moderate/severe calcification (OR 1.85, 95% CI: 1.41-2.42) and +1 point for blunt/no stump (OR 1.45, 95% CI: 1.10-1.92). These points were summed together to form the PROGRESS-CTO perforation score (Central illustration).
For each PROGRESS-CTO perforation risk score, the corresponding perforation percentage risk and the proportion of patients falling in that category in the PROGRESS-CTO registry were calculated (Central illustration). The calculated risk percentages for clinical perforation based on the PROGRESS-CTO perforation score ranged from 0.7%-11.2%. In the registry, 41% of patients had a PROGRESS-CTO perforation score of 2-3, corresponding to a perforation risk of 1.7%-4.6%, and 7.4% of patients had a score of 5, corresponding to a perforation risk of 11.2%.
In our study, large vessel perforations (40%) were the most common clinical perforations followed by distal vessel perforations (26%), epicardial collateral perforations (11%) and septal collateral perforations (7%). The validation of the PROGRESS-CTO perforation score on the different types of perforation showed acceptable performance on ROC analysis for all types of perforation except septal collateral perforation, and it had the highest predicting capability for large vessel perforation (Supplementary Figure 2).
In our study, 61 of the clinical perforations were classified as Ellis Class 1, 120 were classified as Ellis Class 2 and 107 as Ellis Class 3. The validation of the PROGRESS-CTO perforation score on the different types of Ellis Class perforation showed acceptable performance on ROC analysis for all three types of clinical perforation, with increasing AUC for higher perforation severity (Supplementary Figure 3).
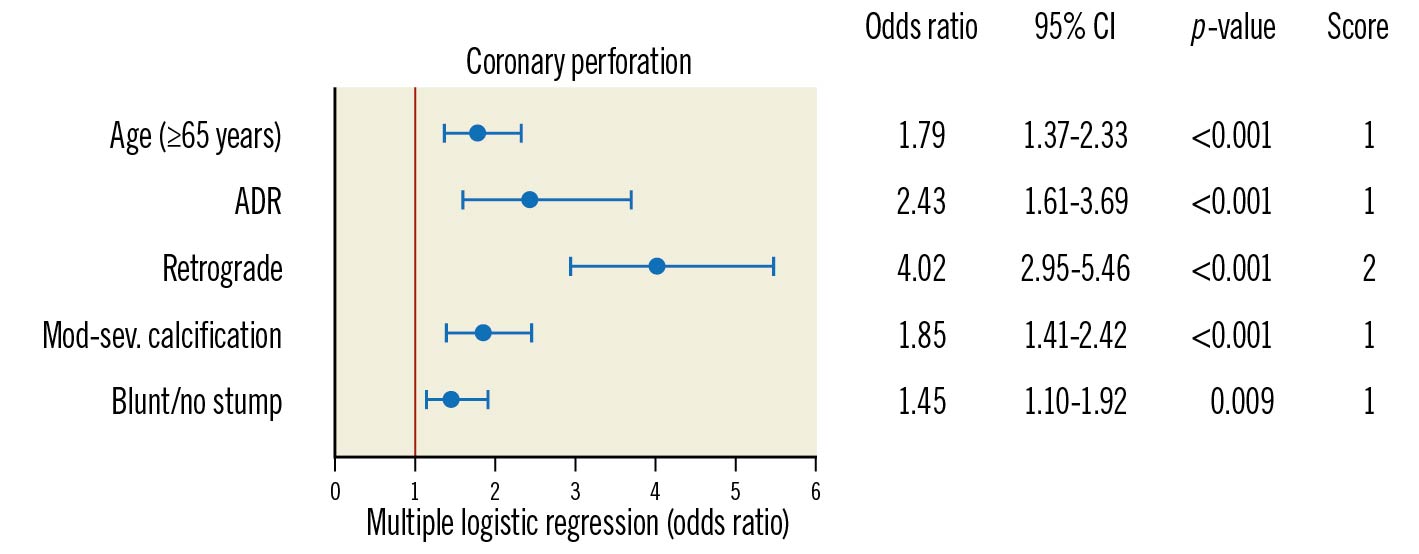
Figure 2. Multiple logistic regression analyses and attributed risk score for the PROGRESS-CTO perforation score. ADR: antegrade dissection and re-entry; CI: confidence interval; Mod-sev.: moderate to severe
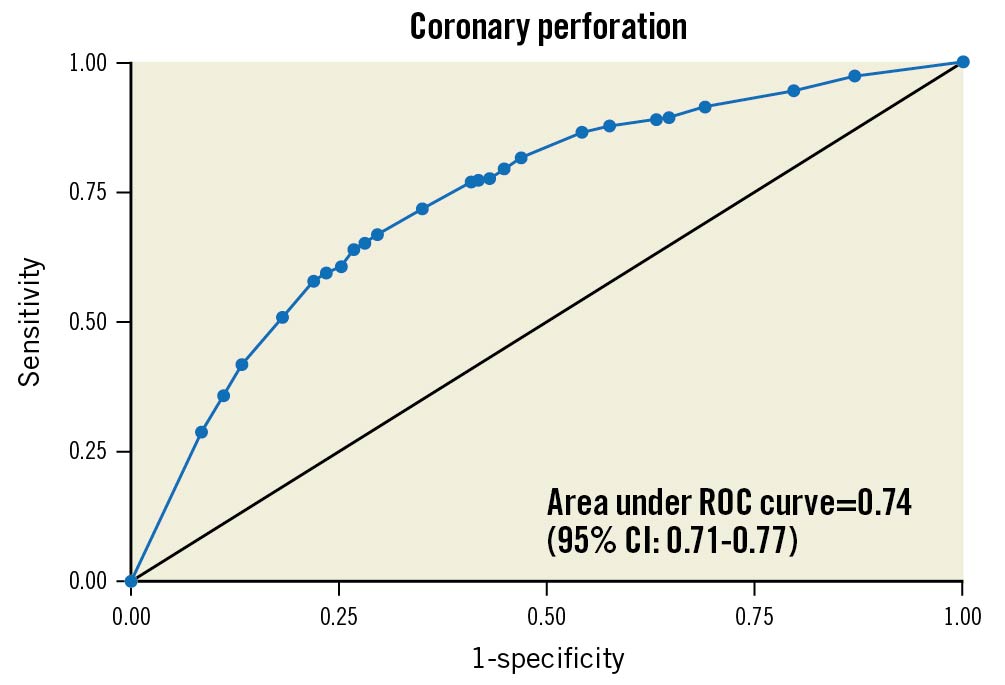
Figure 3. Area under the receiver operating characteristics curve for perforation risk model. ROC: receiver operating characteristic
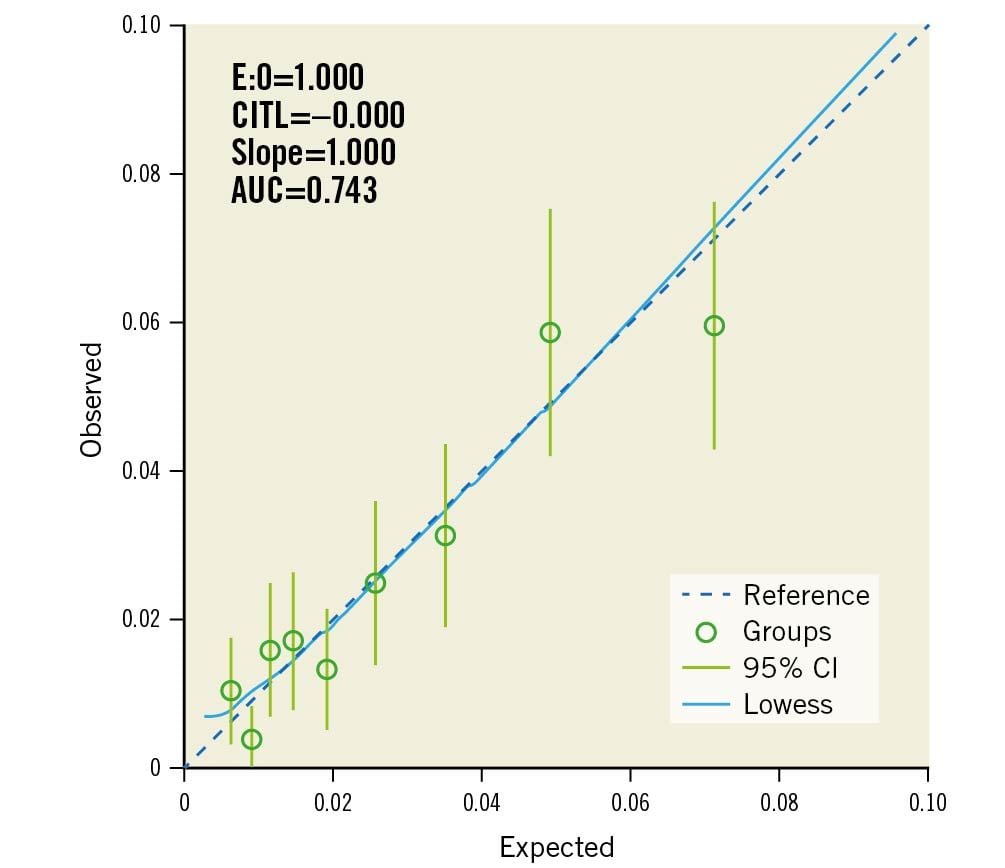
Figure 4. Calibration plot for PROGRESS-CTO perforation score. AUC: area under the curve; CITL: calibration-in-the-large; E:O: expected/observed ratio
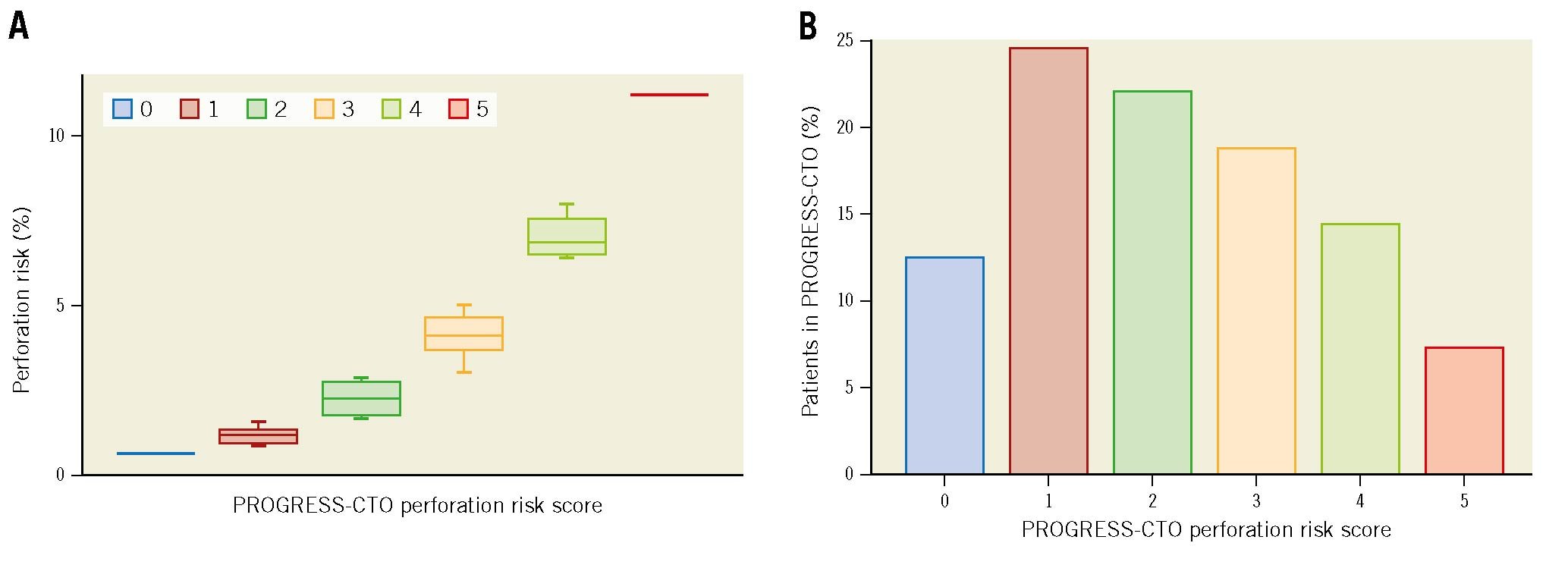
Central illustration. The PROGRESS-CTO perforation score. A) PROGRESS-CTO perforation risk score and corresponding risk percentage. B) Proportion of patients within each perforation risk group. PROGRESS-CTO: Prospective Global Registry for the Study of Chronic Total Occlusion Intervention
Discussion
Our study identified several risk factors for coronary artery perforation in patients undergoing CTO PCI and created and internally validated the PROGRESS-CTO perforation score (clinical perforation prediction scoring system with a maximal score of 5). The five variables of the score are: age (≥65 years=1 point), moderate/severe calcification (1 point), blunt/no stump (1 point), use of ADR (1 point), and use of the retrograde approach (2 points).
The most recent American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines for coronary artery revascularisation downgraded the recommendation for CTO PCI from class 2a to 2b11. While expert operators can achieve technical success rates exceeding 85%, minimising feared complications, such as perforation, is essential, considering that the main indication for CTO PCI is alleviation of symptoms121314.
In our study, patients with clinical coronary artery perforation were more likely to experience adverse outcomes, such as death, tamponade requiring pericardiocentesis, and repeat PCI, which is consistent with the existing literature5615. Understanding the mechanisms of perforation and being able to predict and hopefully avoid perforations could enhance the safety of CTO PCI.
In the present manuscript, we provide a risk score that allows estimation of the risk of clinical coronary artery perforation. We also provide risk percentage intervals corresponding to each score and the proportion of patients falling in each of the risk scores within the PROGRESS-CTO registry (Central illustration). We also provide a calculator with continuous outcomes (age) to allow a more individualised risk calculation tool (Supplementary Table 2).
Hirai et al16 analysed data from 1,000 consecutive patients enrolled in a 12-centre prospective CTO PCI registry (OPEN-CTO: Outcomes, Patient health status, and Efficiency iN Chronic Total Occlusion hybrid procedures) and developed the OPEN-CLEAN (OPEN-CABG, CTO Length, Ejection fraction<50%, Age, CalcificatioN) perforation score for estimating the risk of angiographic perforation during CTO PCI. The OPEN-CLEAN CTO perforation score is computed from 5 parameters: age, prior CABG, CTO length, ejection fraction (EF), and the presence of severe calcification in the lesion. In contrast with the OPEN-CLEAN perforation score, our analysis did not show any independent association between coronary perforation and prior CABG, CTO length, and EF.
The performance of the OPEN-CLEAN perforation score for predicting the risk of angiographic perforation during CTO PCI was recently assessed in the PROGRESS-CTO registry17. The main finding was that the OPEN-CLEAN perforation score was positively associated with the risk of perforation during CTO PCI, with ROC analysis demonstrating an AUC of 0.62 (95% CI: 0.59-0.65), which was lower than the AUC of 0.75 reported by Hirai et al16. The PROGRESS-CTO perforation score showed higher performance on ROC analysis (AUC: 0.741, 95% CI: 0.712-0.773) for predicting the risk of clinical coronary perforation (Figure 3). These differences in risk model performance across diverse cohorts may reflect differences in practice patterns.
Similar to the OPEN-CLEAN perforation score16, our analysis demonstrated that older age and moderate-severe calcification were independently associated with higher risk of perforation. Coronary perforation is more common during CTO PCI of elderly patients, most likely due to a high prevalence of comorbidities, higher complexity of the CTO lesions, and possibly lower tolerance to inadvertent guidewire exits18. Coronary calcification can hinder CTO PCI by impairing wire advancement and penetration, affecting equipment delivery, and preventing stent expansion19. Severe coronary calcification has been associated with lower technical success and higher risk of complications, including perforation20.
Our analysis showed that compared with AW, the use of more advanced techniques such as ADR and the retrograde approach were independently associated with higher risk of perforation. Although the use of these specialised and potentially complex techniques has increased the success rate of CTO PCI, the risk of perforation is also higher, as demonstrated in prior studies21.
Furthermore, our study found an independent association between blunt/no stump in the proximal cap and perforation risk. Possible explanations for this finding are that CTO lesions with blunt or stumpless proximal caps often require the use of higher penetrative force guidewires, or they involve a retrograde approach, which has been associated with higher risk of perforation22. The use of dissection and re-entry techniques, either antegrade or retrograde, increases CTO PCI success rates, especially in very complex cases23, but may also lead to complications, such as donor vessel injury, side branch loss, and perforation of either the CTO target vessel or a collateral vessel24.
Although in some perforation cases no specific management may be needed apart from careful observation, severe perforations require appropriate and emergent treatment with one or more2526 interventions, such as covered stent placement26, embolisation with coils27, fat or other material, thrombin injection28, dissection and re-entry techniques29, and/or emergent pericardiocentesis.
A high PROGRESS-CTO perforation score in a very complex CTO PCI can also help operators identify factors contributing to failure and consider investment techniques as a possible alternative instead of aggressive crossing attempts. Previous studies of “investment” procedures reported high success rates and acceptable complication rates at subsequent attempts30. The PROGRESS-CTO perforation score could, therefore, help operators prepare when performing CTO PCI at high risk of perforation. Also, this score may be useful for quantifying the risk/benefit ratio of each individual patient in order to guide shared clinical decision-making. In some patients, such as elderly patients with unfavourable anatomic characteristics and mild symptoms, CTO PCI may be best not attempted and medical treatment followed instead.
Limitations
First, PROGRESS-CTO is an observational retrospective study without long-term follow-up for all patients. Second, there was no core laboratory assessment of the study angiograms or clinical event adjudication. Third, the procedures were performed at dedicated, high-volume CTO centres by skilled and experienced operators, and these results may not be reproducible by less experienced operators, limiting the generalisability of our findings to centres with limited CTO PCI experience. Fourth, our analysis was performed without adjustment for multiple comparisons.
Conclusions
In conclusion, a score consisting of 1 clinical characteristic (age ≥65 years), 2 angiographic characteristics (moderate/severe calcification, blunt/no stump in proximal cap) and 2 procedural characteristics (use of ADR and use of the retrograde approach) may be useful for predicting the risk of perforation during CTO PCI. This tool can be used to guide procedure planning and clinical decision-making.
Impact on daily practice
Coronary artery perforation is a feared complication of chronic total occlusion percutaneous coronary intervention and often leads to serious adverse clinical events. This study demonstrates that the PROGRESS-CTO perforation score can estimate the risk of clinical coronary artery perforation during CTO PCI. Given the ease of calculation, the PROGRESS-CTO perforation score may be useful for quantifying the risk/benefit ratio and guide clinical decision-making.
Acknowledgements
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the Minneapolis Heart Institute Foundation (MHIF), Minneapolis, Minnesota78. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Funding
The authors are grateful for the philanthropic support of our generous anonymous donors, and the philanthropic support of Drs Mary Ann and Donald A. Sens, Mrs Diane and Dr Cline Hickok, Mrs Wilma and Mr Dale Johnson, the Mrs Charlotte and Mr Jerry Golinvaux Family Fund, the Roehl Family Foundation and the Joseph Durda Foundation. The generous gifts of these donors to the Minneapolis Heart Institute Foundation's Science Center for Coronary Artery Disease (CCAD) helped support this research project.
Conflict of interest statement
K Alaswad is a consultant and speaker for Boston Scientific, Abbott Cardiovascular, Teleflex, and CSI. F.A. Jaffer has done sponsored research for Canon, Siemens, Shockwave, Teleflex, Mercator, and Boston Scientific; is a consultant for Boston Scientific, Siemens, Magenta Medical, IMDS, Asahi Intecc, Biotronik, Philips, and Intravascular Imaging Inc; has equity interest in Intravascular Imaging Inc, DurVena; and the right to receiveroyalties through Massachusetts General Hospital licensing arrangements with Terumo, Canon and Spectrawave. J.J. Khatri has received personal honoraria for proctoring and speaking from Abbott Vascular, Asahi Intecc, Terumo, and Boston Scientific. J.W. Choi is on the advisory board of Medtronic. W.A. Jaberhas received fees from Medtronic,and has received proctoring fees from Abbott. S. Rinfret is a consultant for Abbott Vascular, Abiomed, Boston Scientific Corporation, SoundBite Medical, and Teleflex. W. Nicholson is a proctor and is on the speakers bureau and advisory board for Abbott Vascular, Boston Scientific, and Asahi Intecc; he reports intellectual property with Vascular Solutions. M.P. Patel reports consulting honoraria from Abbott, Medtronic, Terumo, and Cardiovascular Systems Inc. E. Mahmud is a consultant for Abiomed, Medtronic, and Boston Scientific. R.E. Davies has received honoraria from Asahi Intecc, Boston Scientific, Medtronic, and Siemens Healthineers, in addition to being a member of an advisory board for Shockwave Medical. J.L. Kerrigan has received consulting honoraria from Abiomed, Asahi Intecc, Biotronik, Cordis, iSchemaView Inc, Osprey Medical, Penumbra, Philips, and Teleflex. N. Abi-Rafeh is a proctor for and has received speaker honoraria from Boston Scientific and Abbott Vascular. A.M. ElGuindy has received consulting honoraria from Medtronic, Boston Scientific, Asahi Intecc, and Abbott; proctorship fees from Medtronic, Boston Scientific, Asahi Intecc, and Terumo; and educational grants from Medtronic. M.N. Burke has received consulting and speaker honoraria from Abbott Vascular and Boston Scientific. E.S. Brilakis has received consulting/speaker honoraria from Abbott Vascular; is the associate editor of Circulation for the American Heart Association, Amgen, Asahi Intecc, Biotronik, Boston Scientific; is on the board of directors of Cardiovascular Innovations Foundation, ControlRad, CSI, Elsevier, GE Healthcare, IMDS, Infraredx, Medicure, Medtronic, Opsens, Siemens, and Teleflex; has received research support from Boston Scientific and GE Healhcare; is the owner of Hippocrates LLC; and is a shareholder of MHI Ventures, Cleerly Health, and Stallion Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

