Abstract
Aims: Transcatheter aortic valve replacement (TAVR) is established as a treatment strategy for patients with end-stage aortic stenosis, many of whom are suffering from severe pulmonary hypertension (PH). In cardiac surgery patients, PH is associated with less symptomatic improvement and increased late mortality. This study elucidates the impact of PH on outcome after TAVR.
Methods and results: Pre and 90 days post-TAVR, pulmonary artery systolic pressure (PASP) was determined non-invasively by echocardiography in 353 patients undergoing TAVR. PH was classified as absent (<30 mmHg), mild-to-moderate (30-60 mmHg), and severe (>60 mmHg). Three hundred and fifty-three patients at high surgical risk, indicated by a logistic EuroSCORE of 26.6±16.5%, underwent TAVR. The severity of PH before TAVR was related to outcome with two-year mortality rates of 13.9%, 27.3%, and 48.4% for PASP <30 mmHg, 30-60 mmHg, and >60 mmHg, respectively (p=0.001). In patients with baseline PASP >60 mmHg, PASP decreased from 65.6±7.6 mmHg to 49.5±14.0 mmHg (p<0.001) at 90 days after TAVR. Patients with persistent severe PH had a worse prognosis than patients with a decrease of PASP below 60 mmHg (two-year mortality rate: 50.0% vs. 18.6%; p=0.001).
Conclusions: Severe pulmonary hypertension predicts adverse outcome after TAVR. Reduction of PASP after the procedure is associated with favourable prognosis.
Abbreviations
CI: confidence interval
ESV: end-systolic volume
EuroSCORE: European System for Cardiac Operative Risk Evaluation
HR: hazard ratio
LVEDP: left ventricular end-diastolic pressure
PAR: paravalvular aortic regurgitation
PASP: pulmonary artery systolic pressure
PH: pulmonary hypertension
SAVR: surgical aortic valve replacement
STS: Society of Thoracic Surgeons
TAVR: transcatheter aortic valve replacement
Introduction
Transcatheter aortic valve replacement (TAVR) has increasingly become an alternative treatment strategy for patients with severe aortic stenosis regarded as at high risk or inoperable by open heart surgery1-4. However, pre-interventional risk assessment – especially in inoperable patients – and the decision as to whether a patient with several comorbidities should undergo TAVR or not is often challenging and should not be based solely on risk score evaluation5.
The prognosis of patients with aortic stenosis (AS) and pulmonary hypertension (PH) is poor though not fully understood. In general, left ventricular (LV) dysfunction, mitral regurgitation, and diastolic dysfunction lead to elevated left atrial and pulmonary venous vasculature pressures by the upstream transfer of haemodynamic perturbations as “reactive” secondary PH6. In patients undergoing surgical aortic valve replacement (SAVR), PH affects outcome and is associated with less symptomatic improvement and increased late mortality, respectively, but might be reactive with rapid decline after SAVR in certain patients7,8.
For TAVR patients, several studies have shown that preprocedural PH is an independent predictor of mortality7,9,10. However, these studies did not assess haemodynamic changes after the procedure and the impact of these changes on individual prognosis. Since TAVR also facilitates treatment of patients in end-stage AS, many of whom are suffering from severe PH, the impact of PH on prognosis in these patients has to be further elucidated. Furthermore, it remains undetermined whether TAVR leads to an improvement of pulmonary artery systolic pressure (PASP), which can then be translated into a more favourable long-term outcome.
Methods
PATIENT COHORT
Between January 2007 and December 2011, 353 patients with severe aortic stenosis were included into this prospective study and underwent TAVR at the University Hospital in Bonn, Germany, and at the Glenfield University Hospital in Leicester, United Kingdom. Written informed consent was obtained from all participating patients and the study complied with the Declaration of Helsinki. Approval by both locally appointed ethics committees was obtained. Acceptance for TAVR required consensus by the Heart Team.
TAVR was performed with the third-generation 18 Fr CoreValve prosthesis (Medtronic, Minneapolis, MN, USA) or the Edwards SAPIEN valve prosthesis (Edwards Lifesciences, Irvine, CA, USA). In Bonn, TAVR procedures were guided predominantly by angiography and performed under conscious sedation, whereas general anaesthesia and echocardiographic control were used in the patients undergoing TAVR at Leicester. After TAVR, the severity of paravalvular aortic regurgitation (PAR) was assessed angiographically, echocardiographically, and haemodynamically by calculation of the aortic regurgitation index in both cohorts11,12. All patients were recommended to undergo follow-up echocardiography in the out-patient clinic of the hospital after three to four months in both centres.
The primary endpoint of this study was all-cause mortality at two years. The secondary endpoint was the change of PASP at three-month follow-up after TAVR. Information about the cause of death was obtained from the treating hospital or general practitioner.
ASSESSMENT OF PULMONARY ARTERY SYSTOLIC PRESSURE (PASP)
In transthoracic echocardiography (TTE), the maximum jet velocity of tricuspid regurgitant flow was measured by continuous-wave Doppler. Right ventricular systolic pressure was estimated based on the modified Bernoulli equation and was considered to be equal to the PASP in the absence of right ventricular outflow obstruction. PASP was calculated by adding transtricuspid pressure gradient to mean right atrial pressure (RAP) estimated from inferior vena cava (IVC) diameter and motion during respiration as follows: mean RAP was estimated to be 5 mmHg if there was complete collapse of the IVC during inspiration, or was estimated to be 10 mmHg if the IVC collapse was <50%. If the IVC was dilated, mean RAP was estimated to be 15 mmHg if the IVC collapsed by >50% with inspiration, or was estimated to be 20 mmHg if there was no visible evidence of IVC collapse7,13.
STATISTICAL ANALYSIS
Data are presented as mean±standard deviation if normally distributed or as median and interquartile range [quartile 1/quartile 3] if not normally distributed. Continuous variables were tested for normal distribution with the use of the Kolmogorov-Smirnov test. Categorical variables are given as frequencies and percentages. Continuous variables were tested for differences with the Student’s t-test or the ANOVA test, when comparing more than two groups. For categorical variables, the ![]() 2 test was used for further analysis. The change of PASP and other haemodynamic parameters at three months after TAVR (shown as boxplots stratified according to the severity of PH) was assessed using a paired test.
2 test was used for further analysis. The change of PASP and other haemodynamic parameters at three months after TAVR (shown as boxplots stratified according to the severity of PH) was assessed using a paired test.
Survival in PH groups was analysed with the Kaplan-Meier method and compared by the log-rank test. A stepwise backward Cox multivariate analysis including all variables from Table 1 with probability value <0.20 in each Cox univariate analysis was used to determine independent predictors of two-year all-cause mortality after TAVR.
All tests were two-sided; statistical significance was assumed when the null hypothesis could be rejected at p<0.05. Statistical analyses were conducted with PASW Statistics version 21.0.0.0.0 64-bit (IBM Corporation, Somers, NY, USA).
The investigators initiated the study, analysed the data, and wrote the manuscript. All authors vouch for the data and analysis.
Results
BASELINE CHARACTERISTICS
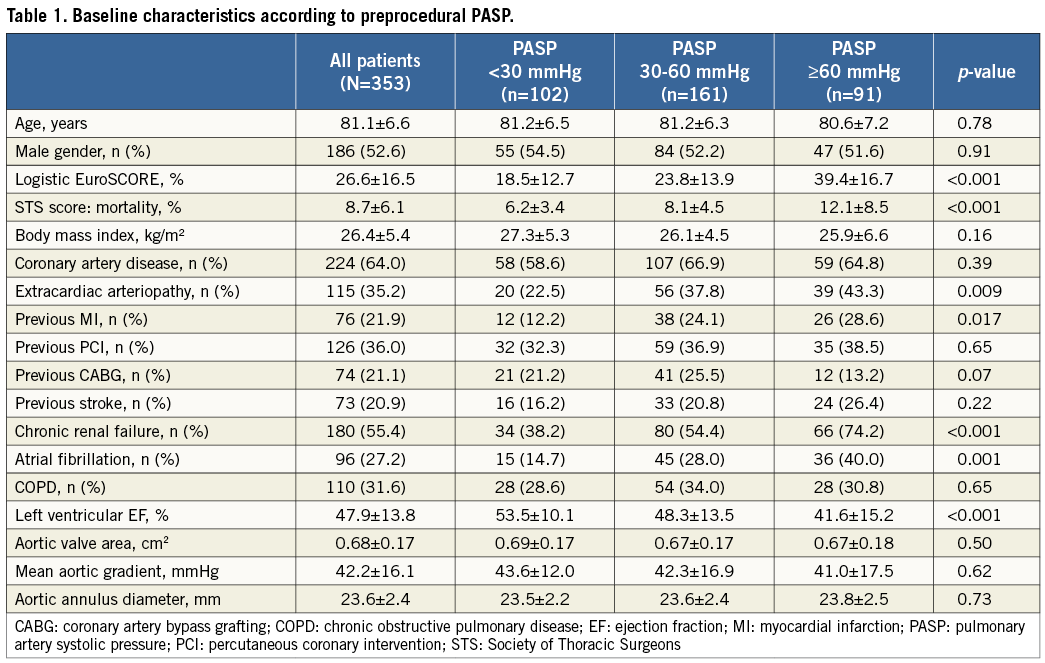
353 patients with severe, symptomatic aortic stenosis (mean age 81.1±6.6 years, 52.6% male, left ventricular ejection fraction [LVEF] 47.9±13.8%) at high risk for open heart surgery, reflected by a mean logistic EuroSCORE of 26.6±16.5% and a STS mortality score of 8.7±6.1%, underwent TAVR by use of either the Medtronic CoreValve (89.0%) or the Edwards SAPIEN prosthesis (11.0%) via the transfemoral (96.0%), or transsubclavian (4.0%) access. Baseline characteristics are summarised in Table 1.
Patients were divided into three groups according to the severity of preprocedural pulmonary hypertension: PASP <30, 30-60, and >60 mmHg. One hundred and sixty-one (45.6%) patients suffered from mild-to-moderate PH (30-60 mmHg), whereas severe PH (>60 mmHg) was found in 91 (25.8%) patients. Patients with mild-to-moderate or severe PH suffered more frequently from comorbidities such as extracardiac arteriopathy (p=0.009), previous myocardial infarction (p=0.017), chronic renal failure (CRF) (p<0.001), and atrial fibrillation (p=0.001). TAVR patients with PASP <30 mmHg had a higher LVEF compared to patients with PASP of 30-60 mmHg or >60 mmHg (53.5±10.1% vs. 48.3±13.5% vs. 41.6±15.2%; p<0.001).
OUTCOME AFTER TAVR
The overall 30-day and two-year mortality rates were 7.1% (25/353) and 28.9% (102/353), respectively. During a mean follow-up time of 517±433 days, the severity of PH before TAVR was significantly related to outcome, with 30-day and two-year mortality rates of 2.0%, 6.8%, or 13.2% (p<0.01) and 13.9%, 27.3%, or 48.4% (p<0.001) for preprocedural PASP <30 mmHg, 30-60 mmHg, and >60 mmHg as assessed by echocardiography, respectively (Figure 1).
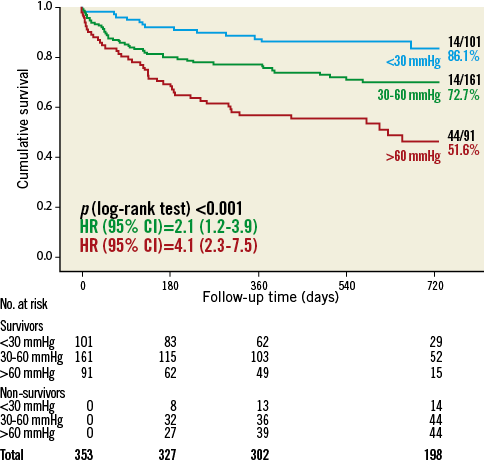
Figure 1. Two-year all-cause mortality according to pulmonary artery systolic pressure (mmHg).
In a multivariate Cox regression analysis of baseline characteristics affecting long-term outcome after TAVR, severe PH was a strong and independent predictor of two-year all-cause mortality with a more than threefold increased mortality risk (hazard ratio [HR] 3.3, 95% confidence interval [CI]: 1.5-7.3; p=0.003) (Table 2).
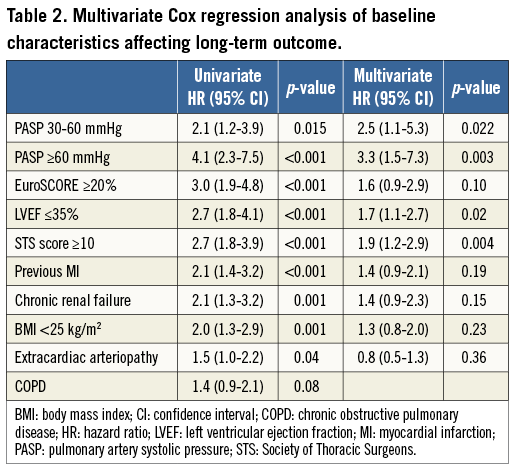
CHANGE OF HAEMODYNAMIC PARAMETERS IN PATIENTS AT 90 DAYS AFTER TAVR
During the first 90 days after TAVR, 46 patients (13.0%) died. Transthoracic echocardiography according to the pre-specified, standardised protocol in the out-patient clinic of the treating hospital (Bonn or Leicester) could be achieved in 240 of 307 survivors (78.2%). Of these 240 patients, 95 patients (39.6%) had a PASP <30 mmHg, 131 patients (54.6%) a PASP between 30 and 60 mmHg, and 14 patients (5.8%) a PASP >60 mmHg. Table 3 demonstrates that 92 of these 240 patients (38.3%) had an improvement of the severity of PH, and that only 31 of 240 patients (12.9%) showed a worsening.
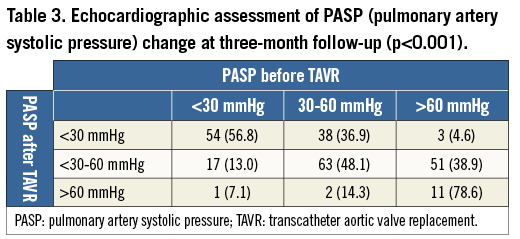
An improvement was shown in 83.1% (54/65) of patients with severe preprocedural PH, with a decrease of PASP below 60 mmHg after the procedure (Table 3). Conversely, only one patient (1.4%) with preprocedural PASP <30 mmHg and two patients (1.9%) with PASP of 30-60 mmHg before TAVR suffered from severe PH at 90 days after the procedure. Patients with persisting or new-onset severe PH at 90 days after TAVR suffered more frequently from more-than-mild PAR than other patients without or with only mild-to-moderate PH (42.9% vs. 8.4% vs. 16.0%; p=0.002) (Table 4). In all three patients with new-onset severe PH, moderate PAR after the procedure was observed. Furthermore, patients with severe PH following TAVR were at higher surgical risk, reflected by logistic EuroSCORE (p<0.001) and STS score (p<0.001), suffered more frequently from chronic renal failure (p=0.03) and extracardiac arteriopathy (p=0.02), and had a lower LVEF compared to patients with PASP <30 mmHg.

In these patients, who suffered from severe PH before the procedure and who were still alive (65 of 91; 71.4%), the mean PASP decreased significantly from 65.6±7.6 mmHg to 49.5±14.0 mmHg (p<0.001) at 90-day follow-up after TAVR (Figure 2A). Patients with persistent severe PH had significantly higher PASP at baseline (71.3±11.1 vs. 64.7±6.1 mmHg, p<0.005) compared to patients with an improvement of PASP after TAVR. In addition, mean LVEF improved from 44.0±15.2% to 50.3±12.4% (p<0.001) (Figure 2B) and mean end-systolic volume (ESV) decreased from 78.0±42.6 mL to 64.5±33.8 mL (p<0.001) in patients with baseline PASP >60 mmHg (Figure 2C). In patients from the Bonn TAVR cohort, NT-proBNP was measured before and three months after the procedure and, in patients with preprocedural PASP >60 mmHg, showed a decrease from 7,925.0 (2,531.8/16,281.3) to 2,992.0 (1,279.0/5,890.0) pg/mL (p<0.001) (Figure 2D).

Figure 2. A) PASP (mmHg) at baseline (N=353) and at 3 months (N=240) after TAVR. B) Left ventricular ejection fraction (%) at baseline (N=353) and at 3 months (N=240) after TAVR. C) End-systolic volume (mL) at baseline (N=324) and at 3 months after TAVR (N=236). D) NT-proBNP* (pg/mL) at baseline (N=194) and at 3 months (N=123) after TAVR. * NT-proBNP was only available in patients from the Bonn cohort. EF: ejection fraction; ESV: end-systolic volume; PASP: pulmonary artery systolic pressure; TAVR: transcatheter aortic valve replacement
IMPACT OF PASP CHANGE ON OUTCOME
Patients with persistent or new-onset severe PH after TAVR had a worse prognosis than patients with a decrease of PASP below 60 mmHg (two-year mortality rate: 50.0% vs. 18.6%; p<0.001). In a landmark analysis of 240 patients (excluding the patients who died during the first 90 days after TAVR or did not undergo the standardised echo protocol), the level of post-procedural PASP was also related to post-procedural long-term outcome (Figure 3): according to a post-procedural PASP <30, 30-60, or >60 mmHg, TAVR patients showed two-year mortality rates of 10.5%, 24.4%, and 50.0% (p<0.001), respectively.
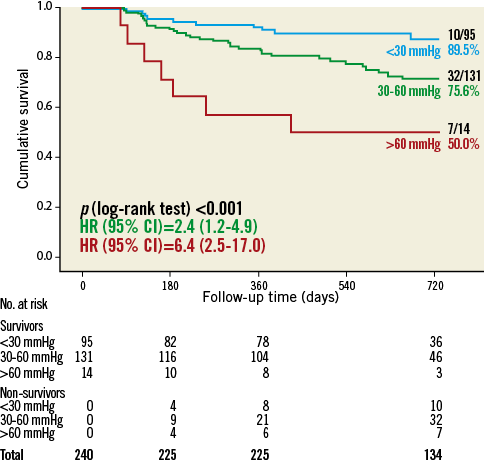
Figure 3. Landmark analysis of 2-year all-cause mortality according to pulmonary artery systolic pressure (mmHg) at 3-month follow-up after TAVR.
Discussion
In this two-centre study, we were able to demonstrate that TAVR led to a significant and sustained decrease of PASP in the majority of survivors at follow-up after three months, which could be translated into a survival benefit compared to patients with persisting or new onset of severe PH. In addition, we confirmed preprocedural PH as a strong and independent predictor of outcome after TAVR. Furthermore, the severity of post-procedural PH, which was associated with the occurrence of more-than-mild paravalvular AR after TAVR, played a pivotal role for outcome in survivors.
We aimed to examine the prevalence and prognostic role of PH estimated by PASP from Doppler echocardiography, which is currently considered the best screening method for PH14. Among heart failure patients, PASP strongly predicts death and provides incremental and clinically relevant prognostic information independently of known predictors of outcomes13. The development of PH in patients with severe aortic stenosis signals a poor prognosis15. Conditions such as aortic stenosis, left ventricular (LV) dysfunction, mitral regurgitation, and diastolic dysfunction lead to increased LV end-diastolic pressure, resulting in elevated pressures in the pulmonary venous circulation and consecutively inducing arterial vasoconstriction and pulmonary arterial remodelling6,13. It has been demonstrated that severe PH is predominantly reactive due to increased LVEDP (Dana Point Class 2)14 and does not represent pulmonary vascular disease, as, e.g., SAVR leads to a rapid decline of PASP in the majority of patients8,15. Consequently, the prognosis of conservatively treated patients is poor7-10,16,17.
In our study population, mild-to-moderate and severe PH was found in 45.6% and 25.8% of the TAVR patients, respectively. These incidences are similar to those reported in the literature for patients with advanced aortic stenosis7,18-20. Several TAVR studies have already described preprocedural severe PH (usually defined as echocardiographically assessed PASP >60 mmHg according to the EuroSCORE definition)21 as one of the major outcome predictors after TAVR9,10,12. Only the study of Ben-Dor et al7 demonstrated that TAVR leads to a decrease of PASP comparable to SAVR. However, the sample size was very small (follow-up echocardiography in 28 of 59 patients) and the authors did not assess the impact of PASP change on echocardiographic parameters and – even more importantly – on outcome. We found a significant decrease in PASP after TAVR combined with a modest increase of LVEF and sustained decrease of ESV and NT-proBNP at follow-up after three months in both cohorts. Importantly, although PH is known to aggravate long-term outcome, TAVR confers a survival benefit for the patient with reduced PASP compared to patients with severe PH post-procedure. The new onset of severe PH was associated with more-than-mild PAR in our patients and might have contributed to the worse outcome in these patients.
Importantly, severe preprocedural PH should not be a contraindication for TAVR, since the prognosis of conservatively treated, inoperable patients with aortic stenosis is even worse16. In our study, patients with a significant postoperative reduction in their PASP level as assessed by echocardiography had a notably better prognosis. Roselli et al demonstrated that many patients undergoing SAVR have at least moderate PH that seems not to be relieved by AVR and is associated with increased mortality, serious complications, and worse late survival8. Although PH severity should be included in risk assessment independently of whether patients undergo TAVR or SAVR, these outcomes, which were confirmed in TAVR patients by us and others, suggest that earlier intervention for aortic stenosis warrants further study.
The debate is ongoing as to why the presence of PH confers such an incremental adverse outcome. In general, the finding of significant PH in TAVR patients may simply signify a greater disease burden6. Our two-centre study also suggests the presence of a more severe comorbidity index in TAVR patients suffering from severe PH before the procedure. However, even after adjustment for several covariates, preprocedural PH remained a strong and independent predictor of outcomes for TAVR patients with graded association between the severity of PH and all-cause mortality. The still high attrition rates of the TAVR procedure in inoperable patients, predominantly attributed to advanced age and comorbidities and not to the procedure itself, will have to be addressed by more appropriate selection criteria, taking into account frailty and functional status in future studies to improve outcomes in this patient group further. Further studies should elucidate which factors contribute to persistent severe PH after TAVR, as these patients might not benefit from the procedure and should not be treated.
Although right heart catheterisation is the gold standard for the diagnosis of PH, we used Doppler echocardiography in this study to screen our patients for PH and to monitor its development after TAVR over time because it is: 1) non-invasive, 2) inexpensive, and 3) widely available. The main disadvantage of right heart catheterisation is that this invasive procedure is associated with some morbidity (up to 1.1%) and mortality (up to 0.055%) and, therefore, the acceptance rate for an invasive follow-up would be very low and render the study cohort incomplete22. In addition, a systematic review of 29 studies recently showed that the correlation of PASP by Doppler echocardiography compared with PASP by right heart catheterisation is acceptable with a summary correlation coefficient of 0.70 and a sensitivity/specificity of 83%/72%, respectively22.
Study limitations
First, the reproducibility and reliability of Doppler in the measurement of PASP are lower than for right-side heart catheterisation22, even though Doppler estimates of PASP have been shown to have adequate correlation with invasive measures23. Secondly, assessment of PASP is variable due to many confounders beyond the control of the diagnostic test, such as volume status, systemic blood pressure, and changes in oxygenation among, e.g., COPD patients23. However, repeated invasive right-side heart catheterisation would not have been feasible. Lastly, a strong selection bias has to be taken into account, since only survivors could be included into the landmark analysis. However, the increased mortality in patients with persisting or new-onset severe PH after TAVR also highlights its relevance for long-term outcome after TAVR.
Conclusions
Severe pulmonary hypertension before TAVR is a strong and independent predictor of adverse periprocedural and post-procedural outcome. However, TAVR is an effective treatment and results in a significant and sustained decrease of PASP in the majority of patients. Patients with reduced PASP after TAVR display a survival benefit compared to patients with persisting or new-onset severe PH.
Funding
J. M. Sinning is supported by a research grant from the University Hospital Bonn (BONFOR). No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, drafting, and editing of the manuscript.
Conflict of interest statement
J. M. Sinning receives research grants from Medtronic and Edwards Lifesciences and speaker honoraria from Medtronic and Edwards Lifesciences. E. Grube has received speaker honoraria from and is proctor for Medtronic. J. Kovac is a consultant with Medtronic and St. Jude and proctor for Medtronic and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

