Abstract
The unsustainable trend of rising healthcare costs necessitates difficult allocation decisions by governments, policymakers, and physicians. Consequently, recent advances in transcatheter valve therapies require not only clinical evaluation, but also careful economic evaluation. Under current indications, each year there are nearly 18,000 new candidates for transcatheter aortic valve implantation (TAVI) in European countries and an additional 9,200 in North America, with an estimated cost of more than $2 billion per year. Nonetheless, when compared with standard medical therapy for severe aortic stenosis (AS), TAVI leads to gains in life expectancy at an incremental cost that is acceptable by most Western standards. On the other hand, for high-risk (but operable) patients with severe AS, TAVI provides no proven survival advantage and only a transient quality of life benefit compared with surgical aortic valve replacement (SAVR). Thus, for these patients, the cost-effectiveness of TAVI compared with SAVR hinges on the magnitude and duration of the quality of life benefit as well as the relative cost of both procedures. Current data suggest that, for patients who are eligible for transfemoral access, TAVI is economically attractive (or even economically dominant) compared with high-risk SAVR. However, the cost-effectiveness of TAVI for patients who are not suitable for a transfemoral approach appears to be less favourable. Transcatheter mitral valve repair is in an earlier stage of clinical implementation than TAVI. As the evidence for this procedure accumulates, more formal economic analysis should be feasible.
Introduction
Over the last several decades, the growth of healthcare expenditure in developed countries has consistently outpaced overall economic growth. Currently, healthcare expenditure represents 10-12% of the gross domestic product in many western European countries and Canada, while this proportion is nearly 18% in the United States1. This unsustainable trend of increasing healthcare costs necessitates difficult resource allocation decisions by governments, policymakers and physicians.
The main drivers of rising healthcare costs are the ageing population and the constant development of costly new technologies2. One of the most promising advances in cardiovascular medicine in recent years has been the development of safe and reliable catheter-based techniques for the treatment of valvular heart disease. In particular, the rapid development and widespread application of transcatheter aortic valve implantation (TAVI) for the treatment of patients with calcific aortic stenosis has raised important questions about the value of these technologies3. Given the high cost of these therapies as well as the growing population of potential candidates, it is clear that therapies such as TAVI require not only clinical evaluation, but also careful economic evaluation. Cost-effectiveness analysis is a formal approach to these issues that seeks to inform both medical decision making and healthcare policy by comparing the benefit of a new therapy with its costs.
In this paper, we will review the current knowledge base regarding economic aspects of transcatheter interventions to treat valvular heart disease. In addition to summarising existing data, we will highlight those areas where the value of a technique or technology is likely to change in the future and suggest fruitful areas for future research.
Burden of aortic stenosis and impact of TAVI on healthcare spending
Aortic stenosis (AS) is the most common valvular heart disease in developed countries, and its burden of disease is expected to increase due to population ageing4,5. A recent systematic review and meta-analysis showed that the prevalence of severe AS in the elderly is 3.4%5. Until recently, surgical aortic valve replacement (SAVR) was the only treatment option in patients with severe aortic stenosis, with approximately 67,500 SAVRs performed each year in the USA alone6. Although obtaining precise estimates of the economic burden of disease is challenging, a recent study projected that there are ~190,000 TAVI candidates in 19 European countries and an additional 100,000 in North America5. At current costs of nearly $70,000 per TAVI procedure (including the associated hospitalisation)7,8, the budget impact of treating all eligible patients would be ~$13.7 billion in Europe and $7.2 billion in North America. Moreover, there are nearly 18,000 new (i.e., incidents) TAVI candidates in Europe and 9,200 in North America annually (Figure 1)5, representing a budget impact of at least $2 billion/year. The budget impact would be substantially larger if the indications for TAVI expand towards low and moderate-risk patients.
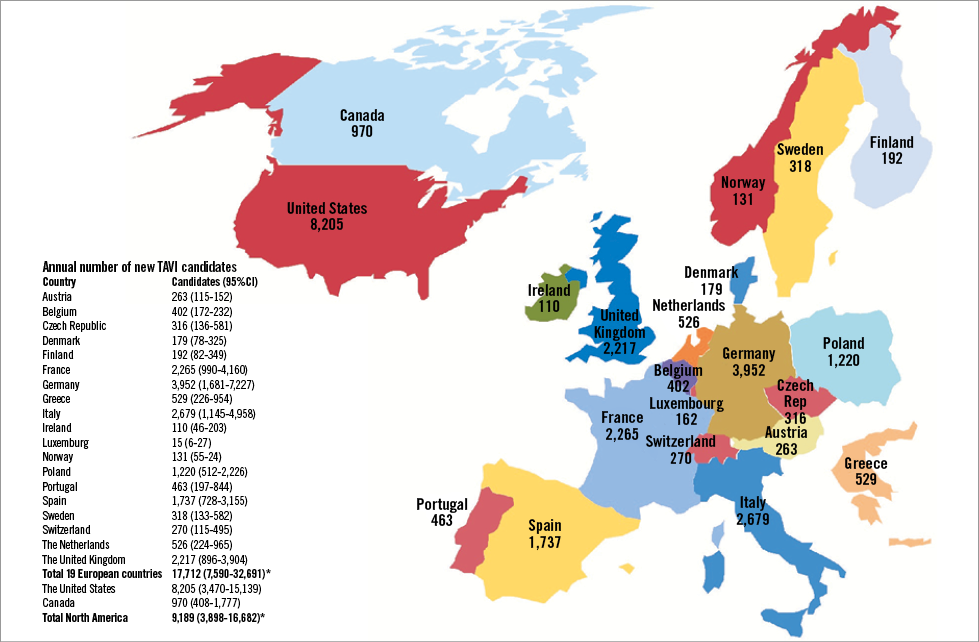
Figure 1. Annual number of TAVI candidates in different countries under the current treatment indications. TAVI: transcatheter aortic valve implantation. Reprinted with permission from Osnabrugge et al5.
Between 2007 and 2011 more than 34,000 patients received TAVI in 11 Western European countries, but there has been substantial variation in the adoption of TAVI among these countries9. The implantation rate in Germany was 88.7 implants per million citizens, while it was 6.1 per million in Portugal. Penetration rates of TAVI display similar inter-country variation, and the estimated overall weighted penetration rate of 17.9% suggests that TAVI is probably still underutilised in some European countries (Figure 2). Economic factors partly explain this variation, since healthcare spending per capita and TAVI-specific reimbursement are associated with higher TAVI use9.
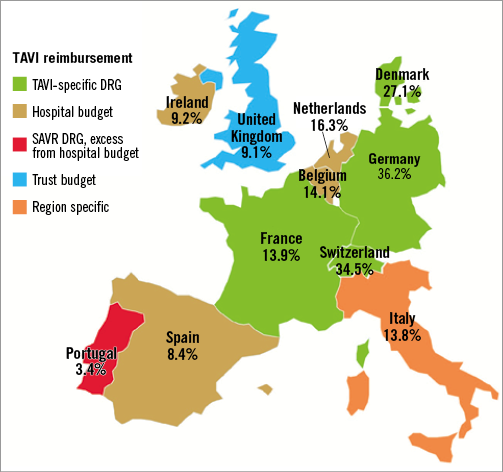
Figure 2. TAVI penetration according to reimbursement systems across Europe. Map of 11 European countries, depicting estimated TAVI penetration rate and the reimbursement system for TAVI that was in place in the year 2011. DRG: diagnosis-related group; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation. Reprinted with permission from Mylotte et al9.
Cost-effectiveness of TAVI versus medical therapy
Several studies have examined the cost-effectiveness of TAVI versus optimal medical management in inoperable patients (Table 1)7, 10-15. The analyses represent a broad range of healthcare systems and incorporate different modelling methodologies, willingness-to-pay thresholds, and discount rates. An individual patient cost-effectiveness analysis based on Cohort B of the Placement of AoRTic TraNscathetER Valves (PARTNER) trial estimated an incremental cost-effectiveness ratio (ICER) of $50,212 per life year gained (Figure 3)7. Other studies have used Markov models (generally based on the aggregate PARTNER B outcomes and survival data) and reported incremental cost-effectiveness ratios ranging from £16,200 (approximately $25,000) per quality-adjusted life year (QALY) gained to $61,889 per QALY10-15. Despite underlying differences in methodology and healthcare systems across these studies, the relatively consistent results led to the conclusion that TAVI is economically attractive compared with medical management in patients who are not candidates for surgery. In other words, the increased life expectancy and quality-adjusted life expectancy after TAVI are achieved at an incremental cost that is, for most countries, within the range of other accepted therapies.

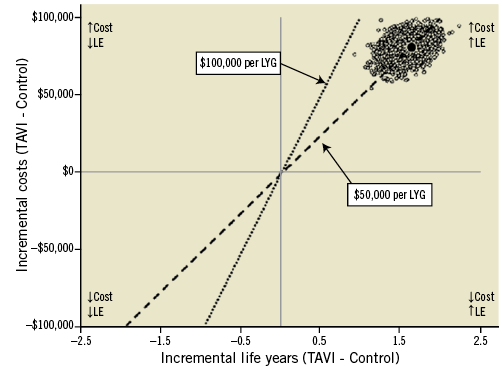
Figure 3. Cost-effectiveness results of PARTNER Cohort B: TAVI versus standard therapy. The mean incremental cost-effectiveness ratio of TAVI versus standard therapy is plotted as the dark circle, along with 5,000 bootstrap replications (cloud of circles). In this cost-effectiveness plane the incremental cost-effectiveness ratio is expressed in US $ per life year gained. The two dashed lines represent two willingness-to-pay thresholds of $100,000 or $50,000 per LYG. LE: life expectancy; LYG: life years gained; TAVI: transcatheter aortic valve implantation Reprinted with permission from Reynolds et al7.
In addition to the general finding that TAVI is reasonably cost-effective for inoperable patients with severe AS, several broad themes have emerged from these studies. The first is that, for patients who are considered inoperable, TAVI results in higher overall healthcare costs. In fact, even if the TAVI prosthesis were provided free of charge, overall healthcare expenditures would be increased7. This finding reflects the fact that inoperable patients with severe aortic stenosis have a relatively short life expectancy (median survival less than two years), which is prolonged substantially if they undergo TAVI. The second factor that underlies this conclusion is the finding that, even after successful TAVI, patients who were otherwise inoperable continue to accrue substantial healthcare-related costs (in the order of $30,000 per year) due to their severe comorbidities. Thus, by extending their lives, the net cost to the healthcare system actually increases.
The second general conclusion to be drawn from these studies is that, in order for TAVI to be cost-effective in inoperable patients with AS, it must result in substantial gains in life expectancy (in the order of one to two years minimum) as well as improved quality of life7. Sensitivity analyses based on the PARTNER trial demonstrate that, if quality of life did not improve after TAVI (but survival did improve), the ICER for TAVI compared with medical therapy would increase to ~$80,000 per quality-adjusted life year (QALY) gained, a value that exceeds societal willingness-to-pay levels in many Western societies16.
Finally, it is important to recognise that whether TAVI is truly “cost-effective” compared with medical management depends on a society’s ability and willingness-to-pay for health benefits. Thus, an ICER of $50,000/QALY (or €30,000/QALY) gained may be acceptable in the United States or relatively wealthy countries in Western Europe but is likely to far exceed the societal threshold in less developed societies where the cost-effectiveness threshold may be <$10,000/QALY gained.
Cost-effectiveness of TAVI versus surgical aortic valve replacement
In Cohort A of the PARTNER trial, 699 patients at high surgical risk were randomised to TAVI via either a transfemoral (TF) or a transapical (TA) approach or SAVR. Over a two-year follow-up period, there was no difference in survival comparing TAVI with SAVR (66.1% vs. 65.0%, p=0.78)17. Thus, in contrast to the results of TAVI in inoperable patients, among high-risk but operable AS patients the main benefit of the less invasive procedure is in quality of life. Indeed, a formal quality of life study conducted alongside PARTNER Cohort A demonstrated that TAVI did result in improved quality of life compared with SAVR in the short term, but that these benefits were restricted to patients who were eligible for a transfemoral TAVI (TF-TAVI) procedure and were limited to the first six months of follow-up18. In contrast, among patients who were only suitable for TA access, there were no quality of life benefits with TAVI compared with SAVR, and indeed there were trends towards worse quality of life at the one and six-month assessments18. Given these findings, it is not surprising that, when these results were expressed in quality-adjusted life years for the purpose of economic analysis, TF-TAVI was associated with a small but significant gain of 0.068 QALYs (95% CI: 0.017-0.1230) over the first year of follow-up, whereas in the TA subset TAVI was associated with a loss of 0.070 QALYs compared with SAVR (95% CI: -0.151-0.012)8. With comparable survival and only small differences in quality of life, costs thus play a pivotal role in the cost-effectiveness of TAVI when compared with SAVR.
Table 2 provides an overview of the published studies that have investigated the cost-effectiveness of TAVI vs. surgical aortic valve replacement (SAVR) for patients at high risk of mortality from SAVR8,10-12,15,19,20. From the US perspective, individual patient cost-effectiveness analysis of Cohort A in the PARTNER trial showed that the TF-TAVI route was an economically attractive strategy compared with SAVR with lower one-year costs and greater quality-adjusted life expectancy (Figure 4A). In contrast, transapical TAVI (TA-TAVI) was associated with higher costs and lower quality-adjusted life expectancy, rendering it both clinically and economically unfavourable relative to SAVR (Figure 4B). Gada and colleagues examined the cost-effectiveness of TA-TAVI using a disease simulation model and also concluded that it was economically dominated by SAVR11.
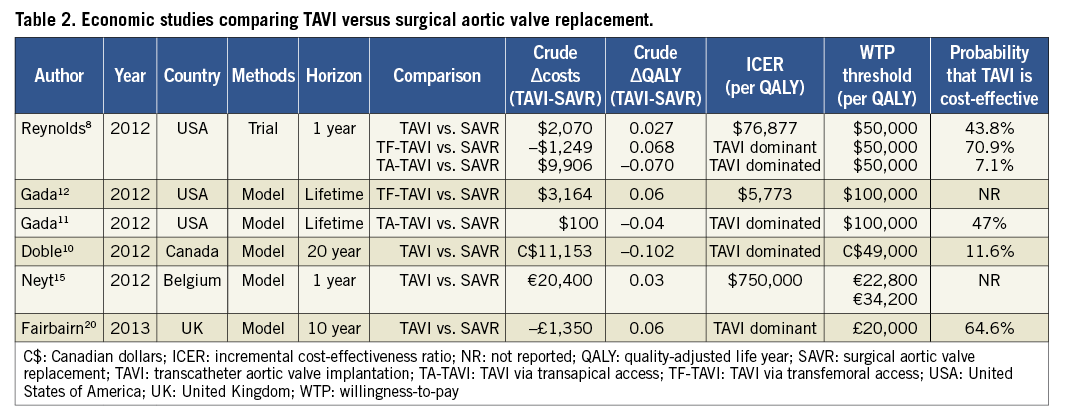
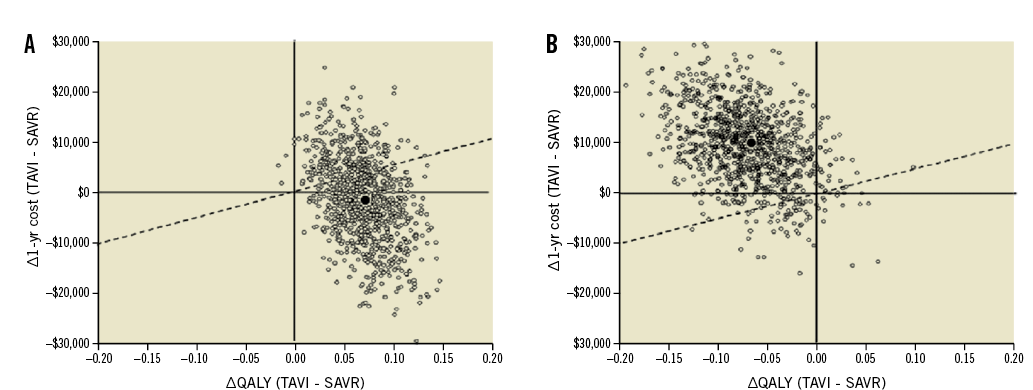
Figure 4. Cost-effectiveness results of PARTNER Cohort A: TAVI versus SAVR. The mean incremental cost-effectiveness ratios for transfemoral (A) and transapical (B) TAVI versus SAVR are plotted as dark circles. The cloud of open circles represents each of the individual 1,000 bootstrap replications based on the observed trial results. The dashed line represents a willingness-to-pay threshold of $50,000 per QALY gained. In this base-case analysis, TF-TAVI was associated with a gain of 0.068 QALYs and cost savings of $1,250 per patient, leading to a position of economic dominance. TA-TAVI was associated with a loss of 0.070 QALYs and a cost increase of $9,906, leading to an economic dominant position of SAVR over TA-TAVI. QALY: quality-adjusted life year; SAVR: surgical aortic valve replacement; TA-TAVI: TAVI via transapical access; TF-TAVI: TAVI via transfemoral access; TAVI: transcatheter aortic valve implantation. Reprinted with permission from Reynolds et al8.
One interesting finding from the available literature is that, even though most model-based cost-effectiveness analyses have used the PARTNER A trial as the key source for their base case assumptions, these studies draw markedly different conclusions that depend largely on the healthcare system in which the analysis was conducted10-12,15,20. For example, one model that incorporated a UK perspective found TAVI to be economically dominant compared with SAVR for high-risk patients20; another model that considered a US perspective found that TF-TAVI was slightly more costly than SAVR but still cost-effective by conventional standards12. However, two studies that considered a Belgian and a Canadian perspective have concluded that TAVI is substantially more costly and only minimally more effective than SAVR, suggesting that TAVI is relatively unattractive from an economic standpoint for patients who are otherwise candidates for SAVR.
By far the most important factor that explains these discrepant results is differences in the cost of high-risk SAVR in various healthcare settings. In countries where high-risk SAVR is quite costly (USA, UK), it appears that the reductions in length of stay following TAVI result in substantial cost offsets to the healthcare system. However, in other settings (e.g., Canada, Western Europe) the costs of SAVR appear to be markedly lower. Whether these differences relate to true differences in healthcare costs across health systems or relate to differences in the types of patients that form the basis for the surgical cost estimates is unclear. Of note, only the US perspective PARTNER trial has specifically captured the cost of high-risk SAVR in patients who would otherwise be considered for TAVI. The rapid acceptance and proliferation of TAVI for high-risk patients (and even intermediate-risk patients) in many European countries has made randomised trials challenging in those settings, leaving unanswered questions regarding the costs and outcomes of SAVR in truly high-risk individuals. Recent population-based studies have suggested that the cost of high-risk SAVR may be lower in some US settings as well21, raising questions about the economic attractiveness of TAVI vs. SAVR in the community setting.
One consistent finding from the available studies is that the cost-effectiveness of TAVI vs. SAVR depends on the access route. In particular, no study to date has demonstrated a favourable ICER for TAVI vs. SAVR among patients who are not suitable for transfemoral access. In general, these findings relate to the observations from the PARTNER A trial that TA-TAVI did not lead to measurable improvements in survival, quality of life or length of stay compared with SAVR18. However, since these results were derived from the very earliest US experience with TA-TAVI, it will be important to revisit these analyses as operator and institutional experience increases and also to assess whether other access routes (e.g., subclavian, direct aortic) might provide more favourable economic outcomes.
TAVI vs. SAVR economics: ongoing challenges and future considerations
Several factors and developments are likely to influence the cost-effectiveness of TAVI compared with SAVR in the future. First, there are currently no long-term follow-up data regarding TAVI durability. Although the biomaterials comprising current transcatheter valves are quite similar to those of current surgical bioprostheses, it is unknown whether the process of crimping and valve deployment might have a harmful effect on the long-term integrity of the valve that could result in higher rates of structural valve deterioration and late reoperation, particularly as TAVI is performed in younger and lower-risk individuals. Although studies have yet to address these issues explicitly, it is intuitive that the additional costs, complications, and quality of life reductions associated with premature valve failure would reduce the cost-effectiveness of TAVI compared with SAVR.
The cost of the TAVI procedure and hospitalisation are also likely to evolve over the next few years. For example, as more manufacturers enter the TAVI market, it is expected that the price of transcatheter valves will drop, making TAVI more cost-effective compared with current levels. Length of stay is another important driver influencing both the cost and cost-effectiveness of TAVI. Although TAVI is less invasive than SAVR, the mean length of stay after TAVI among truly high-risk patients was 10 days in PARTNER A (16 days among patients treated via the transapical approach) and 11 days in a European study of intermediate-risk patients (logistic EuroSCORE of 13)7,8,19. As device profiles continue to decrease and operator experience grows, it can be expected that length of stay for TAVI will decrease, which should also have a favourable impact on the cost-effectiveness of TAVI relative to SAVR (which is a relatively mature procedure that is unlikely to achieve comparable length of stay reductions).
The ongoing SURTAVI (SURgical Replacement and Transcatheter Aortic Valve Implantation) and PARTNER II trials will compare TAVI versus SAVR in intermediate-risk patients (defined as predicted 30-day mortality with SAVR of 4 to 8% based on the STS risk score)22. A cost study in intermediate-risk patients (logistic EuroSCORE of 13) found that TAVI was approximately €10,000 more expensive than SAVR at one year19. This study was, however, based on historical controls and was performed relatively early in the learning curve for TAVI. Demonstration of both economic and quality of life benefits will be an important goal of the two randomised trials in order to justify expansion of TAVI indications into such lower-risk patients.
Cost-effectiveness of transcatheter mitral valve repair
In addition to TAVI, other promising techniques are under development for transcatheter management of valvular heart disease. One such technique is correction of severe mitral regurgitation. The Endovascular Valve Edge-to-Edge REpair STudy (EVEREST) II trial randomised patients with mitral regurgitation to undergo either transcatheter repair or conventional surgical mitral valve repair (or replacement). The study showed that the MitraClip device (Abbott Laboratories, Abbott Park, IL, USA) was less effective at reducing mitral regurgitation, but was associated with superior safety and similar improvements in quality of life at one year23. At four-year follow-up, more patients in the transcatheter repair group had undergone surgery to treat residual/recurrent mitral regurgitation than in the surgical group (24.8% versus 5.5%, respectively; p<0.001)24.
Preliminary results of a patient-level cost-effectiveness analysis of the EVEREST II trial demonstrated that the cost-effectiveness of the MitraClip varied substantially according to the assumed duration of quality of life benefit, the price of the device, and the rate of procedural success25. At one year, the costs for the MitraClip strategy ranged from $37,249 to $53,429, and the QALY gain relative to surgical repair ranged from 0.015 to 0.041, depending on the valve price and trial population analysed. The corresponding one-year costs in the surgical arm were $43,280. The relevance of this analysis to current practice is uncertain, however, since the population of the EVEREST II trial (operable patients with predominantly structural MR) differs substantially from the population in which the MitraClip is currently employed (high-risk/inoperable patients, mainly with functional MR). Data from the ongoing Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for High Surgical Risk Patients (COAPT) and Randomized Study of the MitraClip Device in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation (RESHAPE-HF) trials should provide critical evidence to support the effectiveness of transcatheter mitral valve repair for these indications and should serve as the basis for future quality of life and economic comparisons.
Conclusions
Transcatheter valve therapies are at different stages of innovation and implementation into clinical practice. While TAVI has already been implemented on a large scale, transcatheter mitral valve repair is less widely adopted and has less clinical evidence supporting its use. This difference is reflected in the economic evidence for this therapy. Based on the available data, TAVI can be considered to be reasonably cost-effective (but not cost saving) for patients with severe AS who cannot undergo surgery. With similar survival and only a small benefit in quality of life, the cost-effectiveness of TAVI compared to SAVR hinges mainly on the relative cost of the two procedures as well as the magnitude and duration of the quality of life benefit relative to SAVR. In the USA to date, several studies have demonstrated at least modest cost savings for high-risk operable patients when TAVI can be performed via the transfemoral approach, but substantially higher costs compared with SAVR (driven mainly by the price of the valve) for patients who require transapical access. On the other hand, in many European countries, the cost of SAVR remains substantially lower than that for TAVI (even when performed via the transfemoral approach), raising questions about its economic value in those healthcare settings. The cost-effectiveness of TAVI for high-risk patients remains a moving target; however, progress in valve technology, improved operator experience, and increasing competition among manufacturers are all likely to have a profound impact on the cost-effectiveness of this technology in the future. There are currently only limited data on the economics of transcatheter mitral valve repair. As the clinical evidence for this procedure continues to evolve, additional economic analyses will be warranted.
Conflict of interest statement
M. R. Reynolds has research grant support from Edwards Lifesciences and Medtronic and is a consultant to Medtronic. A. P. Kappetein is a member of the steering committee of the SURTAVI trial, sponsored by Medtronic. D. J. Cohen has research grant support from Edwards Lifesciences, Medtronic, and Boston Scientific and is a consultant to Medtronic. R. L. J. Osnabrugge has no conflict of interest to declare.

