
Over the past decade, the emergence of transcatheter aortic valve replacement (TAVR) has opened a new world of options for treatment of patients with severe symptomatic aortic stenosis (AS). Trials in intermediate- and high-risk surgical patients have demonstrated TAVR to be non-inferior to surgical aortic valve replacement (SAVR)1,2,3. Nonetheless, given the much higher acquisition cost of a TAVR device compared with a surgical bioprosthesis, the cost-effectiveness of this approach has been questioned. While transfemoral TAVR was found to be reasonably cost-effective compared with SAVR in the high-risk population4,5, the economics of TAVR appear to be even more favourable in the intermediate-risk population, for whom transfemoral TAVR is projected both to reduce long-term costs and to improve quality-adjusted life expectancy – an economically dominant strategy6. As a result, TAVR is currently preferred over SAVR in most patients of intermediate or greater surgical risk. Most recently, based on the favourable early results of both the PARTNER 3 and Evolut Low Risk trials, TAVR is now considered a viable approach for nearly all patients with severe AS who have acceptable anatomy for transfemoral TAVR7,8. With expansion into the large population of low-risk patients (who represent more than half of all patients with severe, symptomatic AS9), the cost-effectiveness of TAVR has become even more relevant in many healthcare systems.
In this issue of EuroIntervention, Geisler and colleagues report the results of the first formal cost-effectiveness analysis of TAVR in low-risk patients10.
Using data from the NOTION trial (which randomised 280 low-risk patients to TAVR or SAVR at three Danish centres and followed them for five years11), the authors constructed a decision analytic model in order to project lifetime survival, quality of life (QoL), and costs for low-risk patients treated with either valve replacement strategy. For patients similar to those enrolled in NOTION, they projected that, compared with SAVR, TAVR would increase lifetime medical care costs by 64,561 Danish kroner (DKK) and quality-adjusted life expectancy by 0.09 quality-adjusted life years (QALYs), resulting in an incremental cost-effectiveness ratio (ICER) of 696,264 DKK/QALY – a value that represents intermediate value for the Danish healthcare system (an ICER of less than 375,489 DKK/QALYs would be required to demonstrate high value).
Given these findings, it is important to ask two questions: (1) how confident can we be in these results; and (2) how should these findings affect our choice of valve replacement strategy for low-risk patients with severe symptomatic AS? With respect to uncertainty, the authors provide some insight in the form of sensitivity analyses. Specifically, they note that their results were highly sensitive to variation in long-term mortality after TAVR or SAVR. Moreover, in probabilistic sensitivity analyses (in which all of the modelling parameters were varied simultaneously), they found that the probability that TAVR would be highly cost-effective for the NOTION population was 42%, while the probability that TAVR would be of intermediate economic value was 78%.
However, these results may underestimate the true uncertainty around the cost-effectiveness of TAVR for low-risk patients. One of the fundamental principles of cost-effectiveness analysis is that a treatment that is more costly than its alternative can only be cost-effective if it improves either survival, QoL, or both. Although it is clear from many previous studies that short-term QoL is improved with TAVR compared with SAVR4,5,6, this benefit is generally transient and insufficient to offset any meaningful long-term cost increase. Thus, the cost-effectiveness of TAVR in low-risk patients depends almost entirely on its providing a long-term survival advantage over SAVR – at least in the Danish healthcare system. Whether the NOTION data are sufficiently robust to justify this assumption is highly uncertain. Moreover, the authors do not explicitly consider the long-term consequences of factors such as paravalvular leak (PVL), permanent pacemaker implantation with right ventricular (RV) pacing, and valve durability after TAVR. The degree to which any or all of these factors could impact on long-term costs, QoL and survival in patients treated with TAVR is unknown and could alter the cost-effectiveness of TAVR versus SAVR substantially in a low-risk patient population.
A second consideration in interpreting these results is whether NOTION patients truly represent a “low-risk” population. NOTION enrolled patients with a mean age of 79 years and STS mortality risk of 2.9% – substantially older and sicker than patients enrolled in the PARTNER 3 and Evolut Low Risk trials (mean age 73-74 years, median STS mortality risk 1.9%)7,8. In fact, these baseline characteristics suggest that the NOTION patients were more like the population of the intermediate-risk SURTAVI trial (mean age 79.8 years; median STS risk score 4.5%)12. Thus, while the NOTION cost-effectiveness analysis suggests that TAVR may be reasonably cost-effective for patients in the trial, given the heterogeneity of this population, these findings may be insufficient to convince policymakers that TAVR should be preferred over SAVR in the broader low-risk patient population.
On the other hand, there are several reasons to believe that the NOTION trial may actually have underestimated the value of TAVR. Since NOTION enrolled patients between 2009 and 2013, all TAVR procedures were performed with the first-generation CoreValve® (Medtronic, Minneapolis, MN, USA), and contemporary practices around pre- and post-procedure care were not employed. For example, while the rates of moderate or greater PVL and permanent pacemaker placement with TAVR were 14.5% and 34%, respectively, these rates were only 4.3% and 17.1% in the Evolut Low Risk trial using a newer-generation CoreValve and contemporary preprocedural planning8,11. Moreover, mean length of stay in NOTION (8.9 days) is not representative of contemporary practice13. Given these findings, there is little doubt that, if the NOTION trial were conducted today, the initial cost of TAVR would have been lower and the cost-effectiveness of TAVR would have been more favourable.
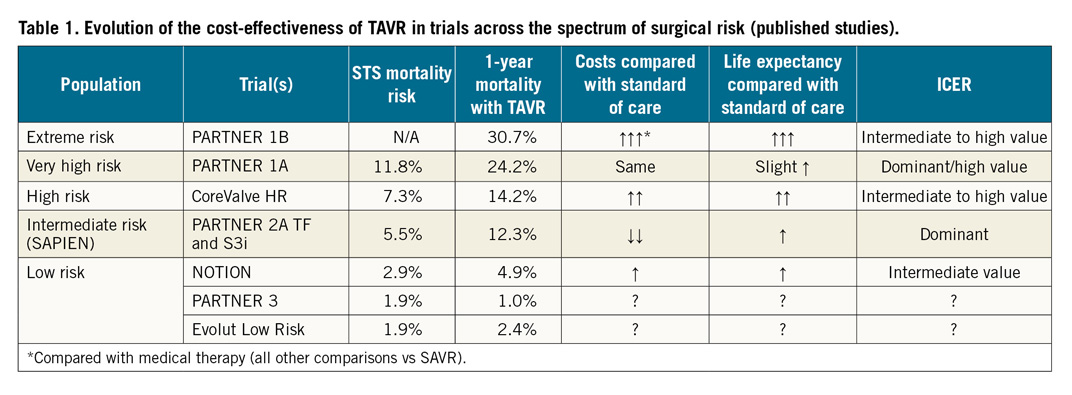
So, what can we conclude from this pioneering study? While the value of TAVR for intermediate- and high-risk patients is relatively well established (Table 1), this study is probably the first of many that will seek to define the cost-effectiveness of TAVR for low-risk patients using more contemporary devices, procedural planning, and intraprocedural and post-procedure care. Given the areas of uncertainty highlighted by the NOTION investigators, future studies will need to focus on several factors including the impact of RV pacing, PVL, and valve durability on both survival and QoL in order to provide a comprehensive evaluation of the cost-effectiveness of TAVR for low-risk patients in current practice. Until these additional studies are completed, however, the results of this study suggest that, by providing both clinical and economic value, the cost-effectiveness of TAVR for low-risk patients may be more than just a notion.
Conflict of interest statement
S.J. Baron is a consultant for Edwards Lifesciences, is on the advisory board of, and has received research support from Boston Scientific Corp. D.J. Cohen has received research support from, and is a consultant for Edwards Lifesciences; received research support from Boston Scientific Corp; received research support from, and is a consultant for Medtronic; and received research support from, and is a consultant for Abbott. The other author has no conflicts of interest to declare.

